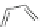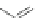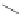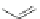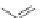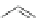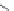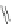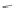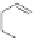Chemistry Reference
In-Depth Information
O
O
Δ
+
O
69%
O
O
23
24
25
Scheme 7 Synthesis of a twisted ribbon 25 [
26
]
R
O
R
R
O
PhI(OAc)
2
,
TfOH, 0
°
C
TMS
TMS
28
TBA
F
R
R=H 82%
R=Me 92%
+
OTf
54%
TMS
I
27
Ph
26
Ph
O
Ph
Ph
Ph
Ph
29
Ph
Ph
Ph
90%
Scheme 8 Synthesis of PAHs 28 and 29 using an aryne precursor [
29
]
O
OTf
Ph
TMS
Ph
Ph
Ph
F
-
78%
TMS
TfO
OTf
TMS
30
31
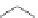
Scheme 9 Synthesis of extended PAH 31 using an aryne Diels-Alder reaction [
30
]
Similarly, the trisaryne equivalent 30 underwent Diels-Alder reaction under
mild conditions with different dienes, yielding various PAHs [
30
]. An impressive
example is shown in Scheme
9
, where 3 new aromatic rings are formed connecting
in total 19 aromatic rings to form product 31.
The synthesis of PAHs was also achieved by an intramolecular Diels-Alder
reaction of an ene-diene stilbene derivative 32 (Scheme
10
) followed by cyclodehy-
drogenation [
31
,
32
].