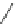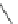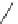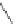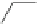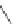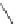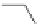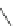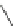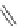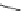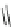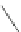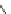Chemistry Reference
In-Depth Information
R
R
R
Cl
Cl
R
Cl
Cl
Pd(PCy
3
)
2
Cl
2
DBU, DMAc
O
O
m
w, 150 ºC
>16%
R=O
n
-C
12
H
25
R
Cl
Cl
R
Cl
Cl
R
R
169
167
168
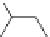
Scheme 53 Synthesis of hexabenzocoronene-based buckybowl 167 [
178
]
Ar
FVP
1100 °C
0.25 Torr
Ar
Ar
3%
Cl
Ar
Ar =
Ar
50
Cl
170
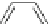
Scheme 54 Synthesis of tubular molecule 170 [
180
]
G
{
¼
G
{
¼
barrier (
ʔ
79.8 kcal/mol) than via a planar transition structure (
ʔ
116.3
kcal/mol).
5.5 Hexabenzocoronene-Based Buckybowl
Buckybowl 169 is not a fragment of common fullerenes, such as C
60
and C
70
(Scheme
53
)[
178
]. The synthesis started with pentacenequinone 167, and the
final step was microwave-assisted Pd-catalyzed cyclization of 168. The maximum
POAV pyramidalization angle of compound 169 was predicated to be 8.4
based on
computational calculations. The bowl-to-bowl inversion barrier of 169 should be
greater than 24 kcal/mol according to the result of a variable-temperature NMR
experiment. It should also be noted that buckybowl 169 easily accepts electrons and
associates strongly with C
70
.