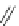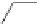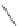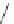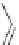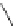Chemistry Reference
In-Depth Information
Cl
Cl
PdCl
2
(PCy
3
)
2
DBU
>10%
1) RhCl(PPh
3
)
3
2) DDQ
Ar
Ar
Cl
Cl
87
90%
Ar=2,6-C
6
H
3
Cl
2
166
165
‡
S
-shaped
Scheme 52 Synthesis and bowl-to-bowl inversion of buckybowl 166 [
177
]
5.3 Tetraindenopyrene
The chemistry of bowl-shaped fragments of C
60
has been intensively investigated,
but that of bowl-shaped fragments of C
70
or higher fullerenes is almost completely
unstudied. Tetraindenopyrene 164, which is a fragment of C
70
[
174
], was prepared
in very low yield from tetraarylpyrene 163 by Pd-catalyzed cyclization
(Scheme
51
). Based on theoretical studies, 164 was predicted to be a shallow
bowl with a very low bowl-to-bowl inversion barrier (0.33 kcal/mol).
5.4 Highly Curved Fragment of C
70
and Higher Fullerenes
Buckybowl 166 is a highly curved subunit of C
70
and higher fullerenes, such as C
76
,
C
78
,C
84
, and others [
175
,
176
]. The studied molecule 166 was easily synthesized
from 1,8-bis(arylethynyl)naphthalenes 87 using a three-step synthetic approach
(Scheme
52
)[
177
]. 7,14-Diarylacenaphtho[
1,2-k
]fluoranthene derivative 165, the
key precursor in this synthesis, was efficiently prepared by simple rhodium-
catalyzed [(2+2)+2] cycloaddition of 87 with acenaphthylene, and subsequently
aromatized by treatment with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ).
The Pd-catalyzed cyclization of 165 gave 166 in low yield. The low solubility of
166 in common organic solvents was mostly responsible for the unsatisfactory
result. The curved structure of 166 was identified by X-ray crystallography and
found to be a rigid bowl-shaped molecule with a deep bowl depth (ca. 2.30
). The
maximum POAV pyramidalization angle of 166 was observed to be ca. 10.8
.
Similar to semibuckminsterfullerene (111-H) [
130
], the bowl-to-bowl inversion of
166 proceeds via a non-planar transition structure. DFT computational studies
indicated that the inversion route via an
S
-shaped transition structure has a lower
Å