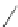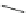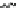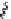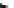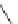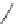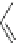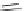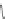Chemistry Reference
In-Depth Information
Br
Cl
FVP
1050 ºC
FVP
1000 ºC
5
-
10%
Cl
7.5
-
9%
Br
Cl
Br
145
144
146
S
S
S
S
S
S
147
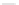
Scheme 45 Synthesis of hemifullerene (145)[
157
,
158
]
acid and subsequently the intramolecular benzannulation efficiently generated
naphthosumanene 141. Similarly, dinaphthosumanenes 142 and trinaphthosumanene
143 were accessed from dibromosumanenes and tribromosumanenes, respectively.
Dibromosumanenes and tribromosumanenes were not formed regioselectively, and
each contains two regioisomers. Therefore, dinaphthosumanenes were obtained as a
mixture of 142a/142b (55:45), but only one regioisomer of tribromosumanenes can
finally give 143. It should also be noted that the bowl-to-bowl inversion barrier for 141
was determined to be 32.2 kcal/mol by an EXSY experiment. Based on theoretical
studies, the bowl-to-bowl inversion barrier and the maximum POAV pyramidal-
ization angle for trinaphthosumanene (143) were suggested to be 63.8 kcal/mol and
10.8
,respectively.
3.3 Hemifullerene
FVP synthesis of
C
3
-symmetric hemifullerene 145 was conducted with chlorinated
tris-fulvene 144 [
157
] or tribenzotriphenylene 146 [
158
] (Scheme
45
). The reaction
mechanism of the latter should involve a radical-mediated cascade cyclization. An
aryl radical, which is generated by bromine atom loss, undergoes 1,2-hydrogen shift
and subsequent closure of the five membered ring. Two types of X-ray-quality
crystals of 145 were obtained from the vapor phase and were verified as ortho-
rhombic (145-
o
) and trigonal (145-
t
)[
159
]. Very recently the association of
hemifullerene (145) with an electron-donating tetrathiafulvalene derivative 147