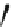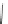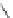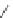Chemistry Reference
In-Depth Information
HO
O
RuCl
3
n
H
2
O
t
-BuOOH
MeMgBr
OH
O
pyridine
CH
2
Cl
2
,40ºC
THF, 0 ºC
56%
73%
O
120
2
OH
121
Scheme 39 Synthesis and reaction of sumanenyl triketone 120 [
139
]
F
S
F
S
SH SH
BF
3
OEt
2
S
py HF, NIS
F
120
CH
2
Cl
2
,25ºC
F
CH
2
Cl
2
,
-
30 ºC
S
64%
24%
S
F
S
F
123
122
Scheme 40 Synthesis and reaction of sumanenyl triketone 123 [
140
]
quenching trianion with CD
3
OD generated trideuterated sumanene
exo
-117 (E
¼
D)
[
137
], which was used to determine the bowl inversion energy (see below).
Like fluorene, the three methylene moieties in sumanene can undergo alkylation
or aldol condensation (Scheme
38
). In the presence of a mixture of aqueous NaOH
and Bu
4
NBr, the reaction of 2 with allyl bromide or 4-methoxybenzyl chloride gave
the corresponding hexa-alkylated sumanenes 118 [
137
]. Similarly, aldehydes
yielded 119 in two regioisomeric forms [
138
].
In the presence of RuCl
3
and oxidant
t
-BuOOH, 2 was converted to triketone 120
in a good yield (Scheme
39
)[
139
]. Consistent with the preferred
exo
attack at
trigonal carbons of sumanene, reaction of 120 with methylmagnesium bromide
selectively gave the product 121 as a single isomer, where all three methyl groups
are at the
exo
positions.
Hexafluorosumanene 123 was prepared from sumanenetrione 120 via cyclic
dithiane 122 (Scheme
40
)[
140
]. Reaction of 122 with pyridine hydrofluoride
(Olah's reagent) in the presence of
N
-iodosuccinimide gave 123 in 24% yield.
The strong electron-withdrawing properties of fluorine substituents cause the first
reduction potential of 123 to be shifted to
1.79 V from
3.21 V (vs ferronce) of
the parent sumanenes (2).
Recently, a new synthetic protocol for synthesis of 9-arylfluorenes or
9,9-diarylfluorenes was reported wherein these products were furnished in good to
excellent yields by Pd-catalyzed cross-coupling reactions of fluorene with haloarenes
[
141
]. This synthetic method was also applied in the preparation of aryl-substituted
sumanenes. Palladium-catalyzed arylation of sumanene (2) was conducted with
1-bromo-2,6-dimethylbenzene, and the desired xylylsumanene 124-xyl was