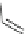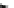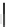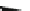Chemistry Reference
In-Depth Information
1) n-BuLi, KOtBu
BrCH
2
CH
2
Br
C
2
H
4
toluene
PCy
3
Cl
Cl
Ru
30%
2) Bu
3
SnCl
3) Cu(2-C
4
H
3
SCO
2
)
47%
Ph
PCy
3
based on
syn-
115
catalyst I
syn/anti-
115
(syn:anti = 1:3)
DDQ
70%
2
116
Scheme 36 Synthesis of sumanene (2)[
134
]
E
t-BuLi
(3 equiv)
CD
3
OD
or SiMe
3
Cl
E
2
E
2
3
−
117
(E = D or SiMe
3
)
Scheme 37 Synthesis of sumanene derivatives [
136
,
137
]
R
Ar
R
X
or ArCHO
R
−
R
or
R
Ar
aq. NaOH, n-Bu
4
NBr
THF, H
2
O, rt
R
2
R
118
119
Ar
Scheme 38 Synthesis of sumanene derivatives 118 and 119 [
137
,
138
]
structure oxidatively to obtain the designed product. The successful synthesis
started with trimerization of norbornadiene to give a mixture of
syn
- and
anti
-115
(ratio 1:3) in a total yield of 47% (Scheme
36
). Treatment of
syn
-115 with catalyst
I under an atmospheric pressure of ethylene furnished hexahydrosumanene 116 in
30% yield (based on
syn
-115) by ring-opening metathesis and subsequent ring-
closing metathesis reactions. Finally, 2 was obtained by the oxidation of 116 with
DDQ.
Generation of the mono-, di-, and trianion of 2 in [D
8
]-THF depends on the
amounts of
t
-BuLi used (Scheme
37
)[
136
]. On the addition of 3 equiv. of
t
-BuLi,
and subsequent treatment with excess amounts of Me
3
SiCl, 2 was converted to tris-
(trimethylsilyl) derivative
exo
-117 (E
SiMe
3
) as the sole isomer, in which all
trimethylsilyl groups were selectively introduced at the
exo
-position. Similarly,
¼