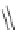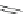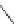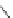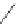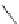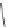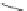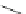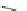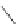Chemistry Reference
In-Depth Information
R
1
R
2
R
2
R
1
R
1
R
2
R
3
SnMe
3
R
4
R
5
Pd(OAc)
2
IPr HCl
RhCl(PPh
3
)
3
t-
BuOK or NEt
3
DME, 110 °C, 3 d
p
-xylene
130 °C, 60 h
Cl
R
3
R
3
Cl
R
3
,R
4
,R
5
=Ar,alkyl,
CMe
2
OH, CHO
19
(R
1
=R
2
=H)
23
(R
1
,R
2
=CO
2
Me, Ph)
R
3
R
3
R
5
R
4
99
100
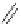
Scheme 29 Preparation of highly substituted indenocorannulenes 100 [
27
]
PdCl
2
(PCy
3
)
2
,DBU
DMAc,170 °C, 30 min
microwave
99%
Cl
98
29
+
101a
102
(13%)
101b
101a
+
101b
(89%, 70:30)
+
103b
103a
103a
+
103b
(48%, 75:25)
104
(35%)
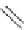
Scheme 30 Synthesis of multi-indenocorannulenes [
89
,
128
]
A rhodium-catalyzed protocol of alkyne cyclooligomerization provides an
alternative route for the preparation of indenocorannulenes 100 [
27
] (Scheme
29
).
The key step of the synthesis starts with 2,3-diethynylcoranulenes 99, which
are accessed by Pd-catalyzed cross-coupling reactions of the corresponding
2,3-dichlorocorannulenes 19/23 with trimethyltin-substituted alkynes. The