Environmental Engineering Reference
In-Depth Information
which is composed of five different subunits (
). The CODH
component of class I/II has two extra clusters (clusters E and F) per monomer,
which presumably facilitate the electron transfer between CODH and a ferredoxin
[
100
]. The function of the
ʱ
,
ʲ
,
ʳ
,
ʴ
, and
ʵ
subunit is still unclear, and it may be involved in
electron transfer from CODH to FAD [
100
].
ʵ
2.3.2 Structural Characterization of Bacterial CODH/ACS
The overall structure and active site of the Ni,Fe-CODH component of bifunctional
CODH/ACS (Figure
12
)[
111
,
112
] are similar to the ones described for
monofunctional Ni,Fe-CODH (Section
2.2
) (Figure
5
). The ACS component con-
sists of three structural domains, which are connected by flexible linker regions [
81
,
111
,
112
]. The N-terminal domain contains a Rossmann fold and interacts with
Ni,Fe-CODH. The active site of ACS, termed cluster A, is a cubane type
[4Fe4S]-cluster bridged to a binuclear Ni,Ni site located in the C-terminal domain
of ACS (Figure
12
).
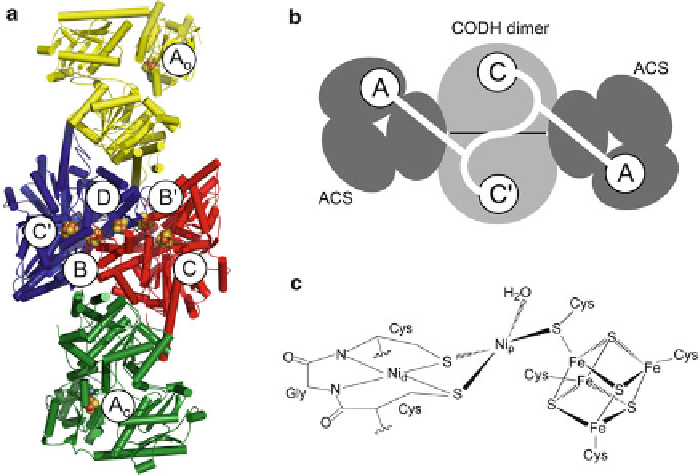
Figure 12 The structure of bacterial CODH/ACS. (a) Cartoon representation of the overall
structure of the
2
CODH/ACS complex from
Moorella thermoacetica
(PDB 1OAO) [
111
].
Metal clusters are presented as balls and sticks and are labeled with A to D. CODH subunits are
colored in blue and red and ACS subunits are colored in yellow for the Ni-Ni-containing cluster A
(A
o
) and green for the Zn,Ni-containing cluster A (A
c
). (b) Schematic representation of the gas
channel connecting the CODH/ACS active sites. (c) Schematic representation of the Ni-Ni
containing cluster A, based on PDB 1RU3 [
81
]. Details are given in the text.
ʱ
ʲ
2
Search WWH ::

Custom Search