Environmental Engineering Reference
In-Depth Information
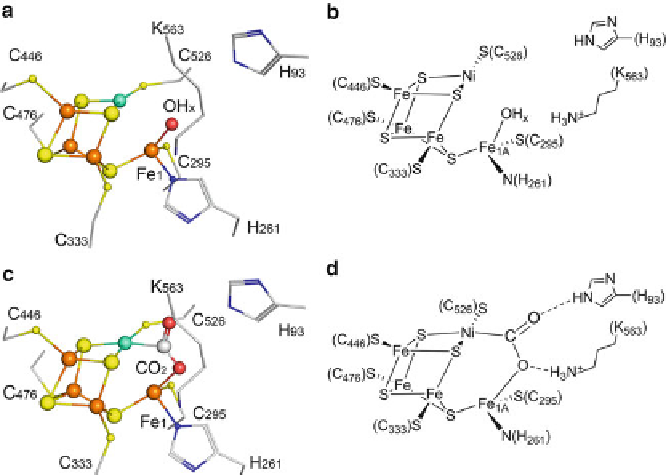
Figure 7 Structure of cluster C from CODH II
Ch
.(a) Ball-and-stick model of active site cluster C
with conserved ligands in the second coordination sphere in the -320 mV state. (b) Schematic
drawing of the [Ni4Fe4S-OH
x
] cluster C. (c) [Ni4Fe4S(CO
2
)] cluster observed in the -600 mV
+CO
2
state. (d) Schematic view of cluster C with bound CO
2
. The secondary positions of Fe
1
(Fe
1B
) and Cys
295
were omitted in the schematic drawings for clarity.
approximately 133
. The Ni,Fe-bound CO
2
is additionally stabilized by hydrogen
bonds to a lysine and a histidine residue [
94
].
2.2.3 Pathways and Channels Involved in Catalysis
The high turnover number of Ni,Fe-CODHs demand a rapid and guided channeling
system for substrates and products. CO and CO
2
, water as well as protons and
electrons must be able to rapidly reach and egress from the active site.
Four different paths reach out from cluster C: a gas channel (CO/CO
2
), a proton
relay, a water network, and an electron transfer chain (Figure
8
). Monofunctional
Ni,Fe-CODHs employ two sets of gas channels. One is conserved and coincides with
the tunnel that is connecting cluster C of Ni,Fe-CODH and the active site cluster A of
ACS in bifunctional Ni,Fe-CODHs (Section
2.3
). The other channel is unique to
monofunctional Ni,Fe-CODHs and is directed to the solvent, allowing rapid progress
and egress of CO/CO
2
from the active site towards the solvent [
95
]. Recently,
molecular dynamics and density functional theory calculations pointed to an addi-
tional, dynamically formed gas channel, through which CO
2
may diffuse from the
solvent to cluster C of CODH/ACS [
96
]. Simulations imply, that upon CO
2
reduction
the extended hydrogen network prohibits CO leakage through the gas channel.
Search WWH ::

Custom Search