Environmental Engineering Reference
In-Depth Information
Crystal structures of monofunctional Ni,Fe-CODHs isolated from
C. hydrogenoformans
(CODH II
Ch
)[
82
] and
R. rubrum
(CODH
Rr
)[
83
] have
been determined. The overall structure is a mushroom-shaped homodimer with
five metal clusters, of which three are cubane-type [4Fe4S] clusters (two cluster B
and one cluster D) and two are the active site clusters C (Figure
5
).
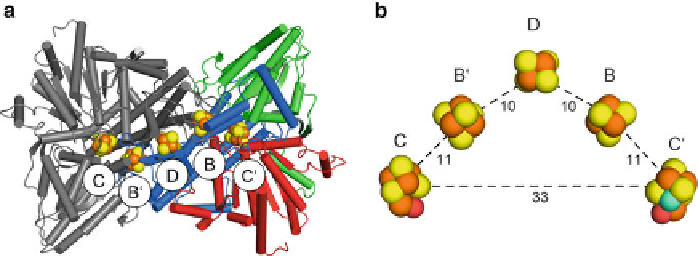
Figure 5 Homodimeric structure of monofunctional CODH II
Ch
.(a) Cartoon-representation of
dimeric Ni,Fe-CODH. The two subunits of CODH are shown with different colors, where one
subunit is highlighted in blue, green, and red for the N-terminal, middle and C-terminal domain,
respectively, and the other in grey. The metal clusters encountered are depicted as spheres (Fe is
colored in orange, S in yellow, Ni in cyan, and O in red). (b) Cluster arrangement in CODH. Cluster
D is connecting the two subunits covalently and is in electron transfer distance to clusters B and B'.
Cluster C/C' is situated on the end of the electron transfer chain, in close distance to cluster B of the
opposing subunit. The distances between Fe atoms of individual clusters are given in
Å
ngstrom.
Cluster D is coordinated by two cysteines of each monomer at the dimer
interface covalently linking both monomers. Cluster B is positioned within typical
biological electron transfer distances [
54
,
84
]of10
Å
from cluster D and 11
Å
from
the active site cluster C.
2.2.2 Electronic States and Structure of Cluster C
Ni,Fe-CODHs catalyze the oxidation of CO as well as the reduction of CO
2
efficiently. CO oxidation at cluster C of CODH II
Ch
occurs with a
k
cat
of
31,000 s
1
and a
K
M
for CO of 18
ʼ
M[
34
]. The specificity constant for CO
10
9
M
1
s
1
) is approaching the diffusion limit and the
reaction is fully reversible.
Spectroscopic studies of the catalytic cycle revealed four distinct electronic
states of cluster C (C
ox
,C
red1
,C
red2
,andC
int
)[
74
](Figure
6
). The diamagnetic
and catalytically inactive, oxidized C
ox
-state can be reductively activated at
potentials below -200 mV [
85
] yielding the one-electron reduced C
red1
state
[
74
]. C
red1
is paramagnetic (S
oxidation (
k
cat
/
K
M
: 1.7
¼
1/2 spin state) with EPR signals at
g
av
¼
1.82,
g
av
¼
1.86, as found for the cluster C of CODH/ACS from
M. thermoacetica
(CODH
Mt
), CODH
Rr
,andCODHI
Ch
, respectively [
86
-
89
].
Two electrons derived from CO oxidation are transferred to cluster C, yielding
1.87 and
g
av
¼
Search WWH ::

Custom Search