Environmental Engineering Reference
In-Depth Information
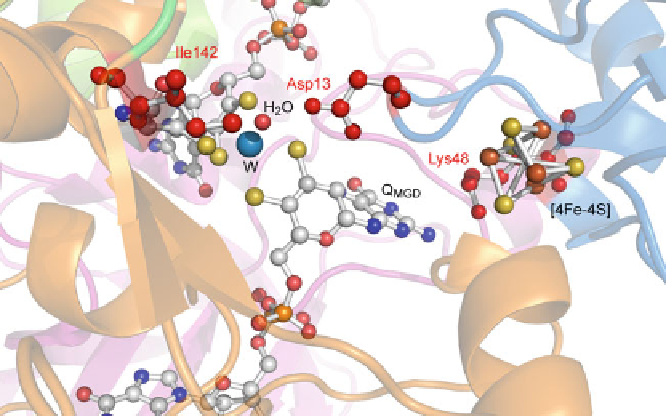
Figure 4 Amino acids exchanged by site-directed mutagenesis. Marked in red: Asp13 that forms
a short hydrogen bond to the water ligand of the W ion was exchanged against alanine and
glutamate, respectively. Lys48 that mediates the electron transfer between the [4Fe-4S] cluster
and the Q
MGD
cofactor was exchanged against alanine. Ile142 that is part of the hydrophobic ring
and involved in positioning the substrate for the reaction was exchanged against alanine.
Three amino acids at the active site could be exchanged by site-directed muta-
genesis: Asp13, Lys48, and Ile142 (Figure
4
). Asp13 forms a hydrogen bond to the
oxygen ligand of the W ion and was expected to be important for the reaction of AH
by helping to activate the oxygen atom for the addition on the C
C triple bond. The
exchange of Asp13 against alanine resulted in a dramatic loss of activity
(0.2
mol min
1
mg
1
for the expressed wild-type) while the exchange of Asp13 against glutamate had
nearly no effect on the activity of AH (2.5
mol min
1
mg
1
ʼ
for the D13A variant compared to 2.6
ʼ
mol min
1
mg
1
for the D13E variant
ʼ
mol min
1
mg
1
for expressed wild-type). These results under-
line the important role of the carboxylic acid group at this position for the reaction
of AH (Figure
5
)[
22
].
Lys48 is located between the [4Fe-4S] cluster and the Q
MGD
cofactor. In other
enzymes of the DMSO reductase family, this residue is involved in electron transfer
between the two cofactors [
29
]. As the reaction of AH does not involve a net
electron transfer, the exchange of Lys48 against alanine did not affect the catalysis
rate of the enzyme (Figure
5
)[
22
].
Ile142 is part of the hydrophobic ring that is expected to form the substrate
binding site at the end of the access funnel towards the active site [
22
]. The
exchange of
compared to 2.6
ʼ
Ile142 against alanine resulted in a strong loss of activity
mol min
1
mg
1
(2.2
ʼ
for
the NarG-AH I142A variant compared to
mol min
1
mg
1
for the NarG-AH fusion protein with the N-terminal chap-
erone-binding sequence). This finding supports the idea that the cavity within the
hydrophobic ring is the substrate binding site of AH (Figure
5
)[
22
].
9.7
ʼ
Search WWH ::

Custom Search