Environmental Engineering Reference
In-Depth Information
is approximately 21 Tg, representing about 80 % of the total global DMS flux to the
atmosphere [
3
].
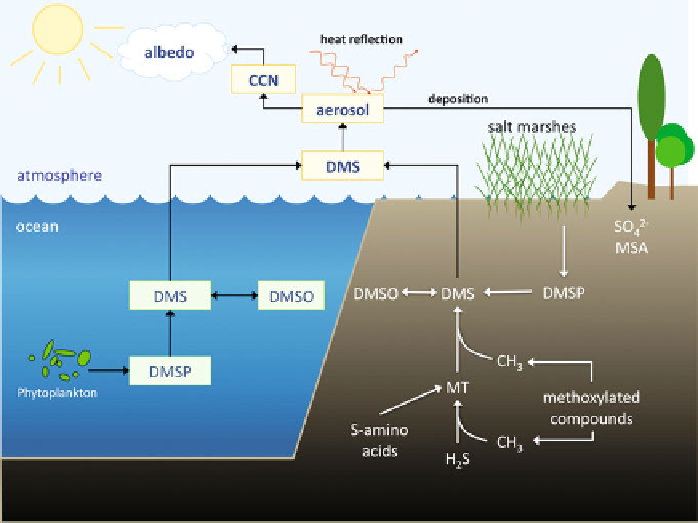
Sea-to-air transfer of DMS is a process that was recognized to provide a missing
link in the global sulfur cycle that is responsible for sulfur transport from the oceans
to the continents (Figure
1
)[
4
]. Following its transfer into the atmosphere, DMS is
oxidized by hydroxyl radicals and nitrate to mainly sulfate and methanesulfonic
acid, which are important components of atmospheric aerosols [
5
,
6
]. Atmospheric
transport of these sulfur compounds and their deposition in terrestrial environments
contribute to maintaining sulfur levels in soils which is important for plant produc-
tivity [
7
,
8
].
Figure 1 Transformations of dimethylsulfide in the biosphere. DMS, dimethylsulfide (CH
3
-S-CH
3
);
DMSO, dimethylsulfoxide (CH
3
-SO-CH
3
); DMSP, dimethylsulfoniopropionate
((CH
3
)
2
S
+
CH
2
CH
2
COO
-
); MT, methanethiol (CH
3
-SH); MSA, methanesulfonic acid (CH
3
-SO
3
H);
CCN, cloud condensation nuclei. Reproduced with permission from [
1
]; copyright 2010, Oxford
University Press.
In addition to its role in atmospheric sulfur transport, the sulfate aerosols derived
from DMS in the atmosphere reflect heat radiation, and in doing so, reduce the
radiative forcing of the atmosphere [
9
]. Furthermore, atmospheric sulfate aerosols
can act as cloud condensation nuclei which may contribute to temperature regula-
tion of the Earth through enhanced cloud cover and albedo formation. It was
suggested that changes in albedo may reduce the incidence of sunlight on the
surface of the ocean with potential consequences for primary productivity. This
potential
link between dimethylsulfoniopropionate (DMSP) production, DMS
Search WWH ::

Custom Search