Environmental Engineering Reference
In-Depth Information
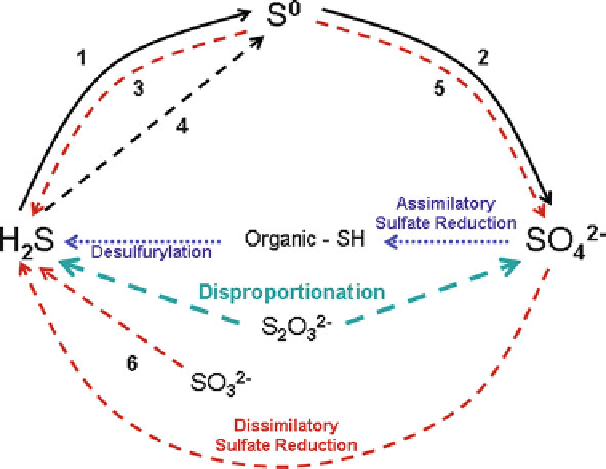
Figure 1 The biological sulfur cycle with roles of bacteria identified. Solid lines indicate aerobic
reactions, dashed lines indicate anaerobic reactions, and dotted lines indicate both aerobic and
anaerobic activity. Desulfurylation by many aerobic and anaerobic prokaryotes, assimilatory
sulfate reduction by many aerobic and anaerobic microorganisms, dissimilatory sulfate reduc-
tion by anaerobic organisms listed in Table
1
of this chapter and in Table
1
of Ref. [
25
], and
disproportionation of thiosulfate by
Desulfovibrio
and
Desulfocapsa
. 1 and 2: Sulfide and sulfur
oxidation by colorless sulfur bacteria. 3: Sulfur reduction by the anaerobic microorganisms listed
in Table
1
of this chapter. 4 and 5: Anaerobic sulfide and sulfur oxidation by purple sulfur bacteria
and green sulfur bacteria. 6: Sulfite-reducing bacteria.
systems. The inorganic sulfur compounds of biological relevance which occur in
the biological sulfur cycle are elemental sulfur, sulfate, sulfite,
thiosulfate,
polythionates, sulfide, and polysulfides (Figure
1
).
Sulfur can adopt many oxidation states, ranging from
2 to +6. Inorganic sulfur
compounds of intermediate oxidation states can serve as electron acceptors or
donors in redox processes. In contrast, sulfate and sulfide cannot be further oxidized
or reduced, respectively, and they are therefore the final products of most sulfur
oxidation or reduction pathways. The biological roles of inorganic sulfur com-
pounds are rather restricted: either they serve as acceptors or donors of electrons for
dissimilatory energy-generating electron transport (almost exclusively among pro-
karyotes), or they are employed as sources for sulfur assimilation, very common in
prokaryotes as well as in algae, fungi, and plants.
Despite its toxicity (5-fold higher than CO), H
2
S is a fundamental molecule in
both anaerobic and aerobic organisms. Since the first description of hydrogen sulfide
toxicity by Ramazzini in 1713, most studies about H
2
S have been devoted to its toxic
effects with little attention paid to its physiological function [
1
]. The liberation of H
2
S
is controlled not only by the rate of its production by sulfate- and sulfur-reducing
prokaryotes, but also by its tendency to rapidly precipitate as metal sulfides, its
Search WWH ::

Custom Search