Environmental Engineering Reference
In-Depth Information
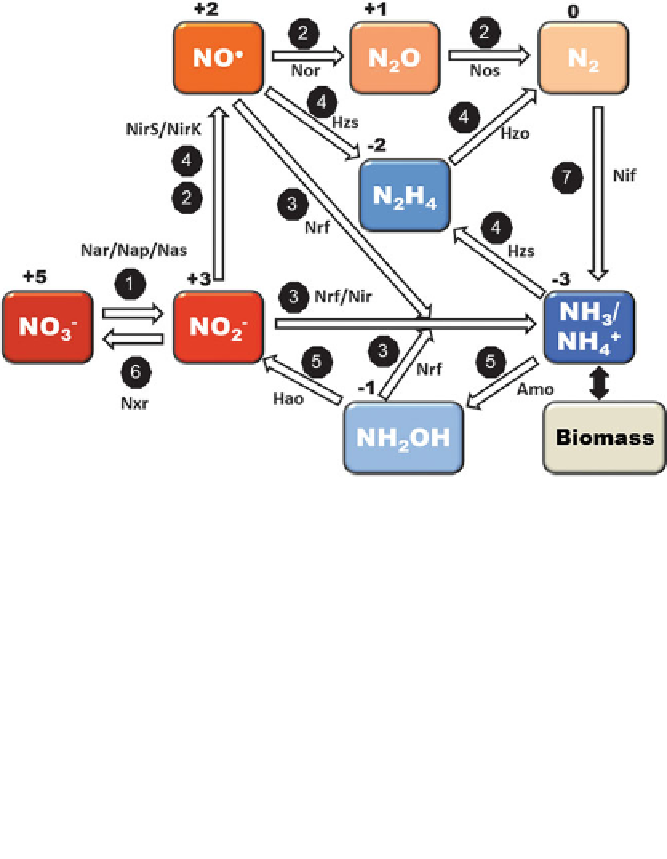
Figure 1 Conversion of nitrogen compounds serving as substrates in nitrogen cycle processes
relevant to this article. The oxidation state of nitrogen atoms is indicated above the boxed
compound. Numbers in black decagons refer to the following processes: 1, respiratory nitrate
reduction to nitrite; 2, denitrification of nitrite to N
2
; 3, Nrf-dependent ammonification; 4, anaer-
obic ammonium oxidation (anammox, i.e., comproportionation of nitrite and ammonium to form
dinitrogen); 5, ammonium oxidation to nitrite; 6, nitrite oxidation to nitrate; 7 nitrogen fixation.
These metabolic pathways are catalyzed by distinct respiratory enzyme systems that are desig-
nated by the following abbreviations: Amo, ammonium monooxygenase; Hao, hydroxylamine
oxidoreductase; Hzo, hydrazine oxidoreductase; Hzs, hydrazine synthase; Nap, periplasmic nitrate
reductase; Nar, membrane-bound nitrate reductase; Nas, assimilatory nitrate reductase; Nif,
nitrogenase; Nir, assimilatory nitrite reductase; NirK, copper nitrite reductase; NirS, cytochrome
cd
1
nitrite reductase; Nor, nitric oxide reductase; Nos, nitrous oxide reductase; Nrf, cytochrome
c
nitrite reductase; Nxr, nitrite oxidoreductase. Modified from [
24
].
important route, the so-called anammox process (
an
aerobic
amm
onium oxidation).
This process occurs in several Planctomycetes and depends on a specialized cell
compartment, the anammoxosome, in which ammonium is converted to N
2
via NO
amount of N
2
per molecule of nitrite consumed as denitrification and does not
necessarily require an external reductant (equation
4
)[
10
-
14
].
NH
4
þ
NO
2
!
N
2
þ
ð
Þ
2H
2
O
4
Naturally occurring dinitrogen gas makes up 78 % (by volume) of the Earth's
atmosphere. However, it usually has to be converted to its biologically useful form,
ammonia, because of its unavailability for most living organisms (equation
5
).
Search WWH ::

Custom Search