Environmental Engineering Reference
In-Depth Information
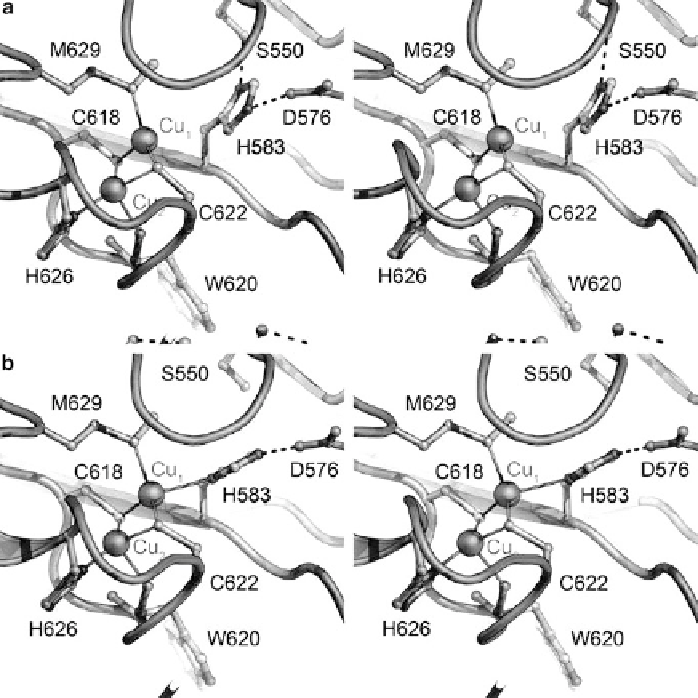
Figure 6 The electron transfer site Cu
A
of N
2
O reductase. Stereo representations of the two
conformations observed in structures of N
2
OR. (a) In purple N
2
OR from
P. stutzeri
, as isolated,
residue His583 was in most cases not a ligand to Cu
1
of the Cu
A
site resulting in a shift of the
copper ion into the plane formed by the two cysteine residues, Cys618 and Cys622, and Met629
(PDB ID 3SBQ). The experimental data is consistent with a sharpening of the EPR hyperfine
structure in this conformation. (b) In all instances with substrate bound to Cu
Z
, the side chain
of His583 was flipped back to coordinate Cu
1
of the Cu
A
center, linking the metal cluster to
the protein surface
via
residue Asp576 to allow for electron transfer from an external donor
(PDB ID 3SBR).
The two Cu ions of Cu
A
show a distorted tetrahedral coordination environment
that represents the classical 'entatic' state between a square-planar geometry with
hard ligands (preferred by Cu(II)) and a tetrahedral geometry with soft ligands
(preferred by Cu(I)) [
63
]. In Cu
A
,
the two cysteine ligands are shared in a
2
-bridging fashion, and each ion in addition has a histidine ligand (Figure
6
).
The coordination environment is complemented in each case by a further ligand
that differs between the two metals. For Cu
1
, this ligand is the oxygen atom of a
backbone carbonyl group, while for Cu
2
the first coordination sphere is completed
ʼ
Search WWH ::

Custom Search