Environmental Engineering Reference
In-Depth Information
c
-type cytochromes. This has been analyzed in
W. succinogenes
[
23
,
36
], and it is
commonly interpreted to originate from a fusion event of the reductase enzyme
with a soluble electron donor. A cytochrome
c
552
of similar architecture serves as
electron donor to the enzyme of
M. hydrocarbonoclasticus
[
37
], while for other
NosZ orthologs the type-I copper protein pseudoazurin was described to also fulfill
this function [
38
]. This functional variability in the architecture of electron transfer
chains is not unprecedented within the pathway of denitrification and a very similar
case was described for cytochrome
cd
1
nitrite reductase [
39
].
As an additional layer of complexity, NosZ itself was found to occur in a series
of forms that are distinct in terms of spectroscopy and reactivity [
40
]. When isolated
in the absence of dioxygen, preparations had a deep purple color, with high catalytic
activity and approximately 8 mol of Cu per mol of dimeric enzyme of
P. stutzeri
[
41
,
42
]. This was designated form I of N
2
O reductase, and it is considered to be the
physiologically relevant active form [
43
], although the copper content at the time
was significantly underestimated. When isolated in the presence of dioxygen,
the enzyme showed reduced catalytic activity and a different optical spectrum.
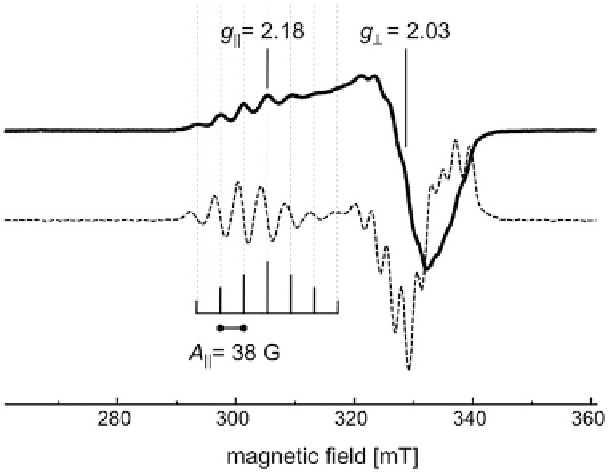
Figure 4 Electron paramagnetic resonance spectrum of N
2
O reductase. In a continuous-wave
EPR experiment at X-band (
9.4 GHz) recorded at 10 K, N
2
OR shows a characteristic axial
signal dominated by the contribution from the Cu
A
site. Both the
g
||
and the
g
⊥
region of the EPR
spectrum are split into a complex hyperfine pattern that was best resolved in the purple form I of
the enzyme where the second metal site, Cu
Z
, was in the [4Cu:2S] configuration. The seven-line
pattern is well resolved in the first harmonic (solid), and the second harmonic (dashed) shows that
both
g
regions are split with an intensity ratio of 1:2:3:4:3:2:1 that is explained by two coupled
copper nuclei (
63,65
Cu nuclear spin
I
ʽ ¼
¼
3/2) in a fully valence-delocalized arrangement.
Search WWH ::

Custom Search