Environmental Engineering Reference
In-Depth Information
1
Introduction: The Biogeochemical Nitrogen Cycle
The bulk elements carbon, oxygen, hydrogen, nitrogen, and sulfur are the
fundamental building blocks for all classes of biomolecules. They are abundant in
our environment and were readily available during evolution to be combined into
molecules of increasing complexity that form the basic classes of biological macro-
molecules: carbohydrates, lipids, amino acids, and nucleic acids. Water is an obvious
and omnipresent source both for oxygen and hydrogen, and nature has devised
various different pathways for the assimilation of carbon, the most prominent being
the light-driven fixation of CO
2
in the Calvin cycle during photosynthesis. The
remaining elements, nitrogen and sulfur, are present in a series of different modifi-
cations and oxidation states that are chemically or enzymatically interconverted in
global, biogeochemical cycles [
1
]. For nitrogen, this cycle spans eight oxidation
levels ranging from+V in nitrate, NO
3
,to-IIIinammonia,NH
3
[
2
,
3
], and only
the latter can be incorporated into amino acids
via
the reactions of glutamine synthase
or glutamate dehydrogenase. Nitrate undergoes a two-electron reduction to nitrite,
NO
2
, catalyzed by a molybdenum-containing nitrate reductase, and nitrite in turn
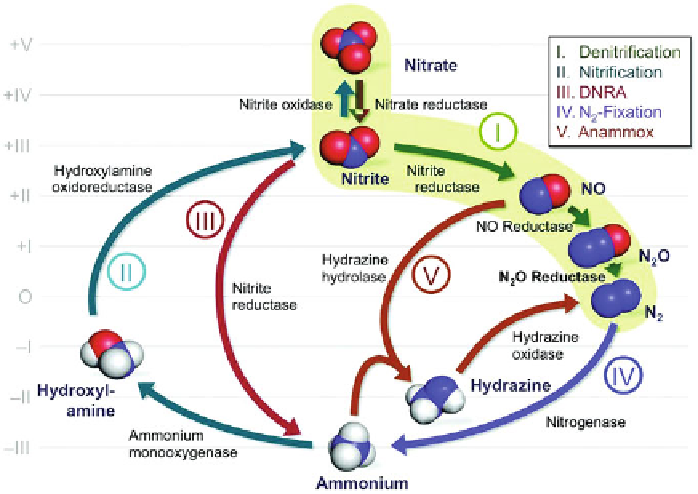
constitutes the central metabolic hub of the nitrogen cycle (Figure
1
).
Figure 1 The biogeochemical nitrogen cycle. A network of reactions catalyzed by
metal-containing proteins connects the different modifications of the element. The pathway of
denitrification (highlighted in green) is a four-step metabolism to reduce nitrate (NO
3
)
via
nitrite
(NO
2
), nitric oxide (NO), and nitrous oxide (N
2
O) to dinitrogen (N
2
). Nitrous oxide reductase
catalyzes the final step of denitrification, and although the reaction is thermodynamically highly
favored, a high activation energy barrier conveys substantial kinetic stability to N
2
O.
Search WWH ::

Custom Search