Environmental Engineering Reference
In-Depth Information
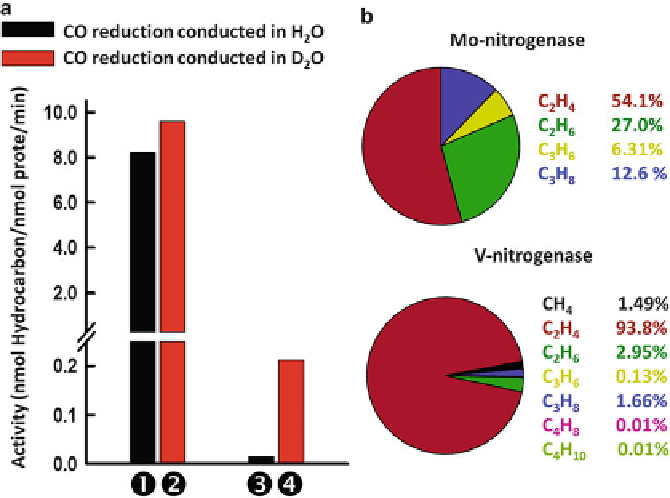
Figure 9 Comparison of total specific activities of CO reduction by Mo- and V-nitrogenases in
the presence of H
2
O and D
2
O(a) and the product distributions of hydrocarbons in the reactions
catalyzed by the two nitrogenases (b). Shown are the total activities of hydrocarbon formation
from CO by Mo- and V-nitrogenases in H
2
O- (
black
) and D
2
O- (
red
) based reactions. In H
2
O,
the V-nitrogenase is 680-fold more active than the Mo-nitrogenase (
versus
); whereas in
➌
⚊
D
2
O, V-nitrogenase is only 50-fold more active than the Mo-nitrogenase (
). The
activity of V-nitrogenase is minimally impacted upon D
2
O substitution (
⚋
versus
⚊
); whereas
the activity of Mo-nitrogenase is significantly increased upon D
2
O substitution (
➌
versus
⚍
).
For calculations of product distributions, the total amounts of hydrocarbons formed in V- and
Mo-nitrogenase-catalyzed reactions were set as 100 %, and the percentages of individual products
were determined accordingly.
⚋
versus
⚍
5 Conclusions
For decades, nitrogenase has remained one of the most enigmatic topics in the field
of metalloprotein biochemistry. From the unveiling of its structure, to the quest of
its reaction mechanism, there are surprises in almost every footstep toward a better
understanding of this complex metalloenzyme system.
While significant strides have been made in elucidating the structure of the
Mo-nitrogenase and the key steps in the reaction of N
2
reduction by this enzyme,
the exact mechanism of nitrogenase is yet to be defined. Much work needs to be
done to address the question of how nitrogenase overcomes the great energy barrier
to cleave the N,N triple bond, as well as the question of how nitrogenase breaks the
isoelectronic C,O triple bond, which may provide valuable, albeit indirect, insights
into the mechanism of N
2
reduction by nitrogenase.
Search WWH ::

Custom Search