Environmental Engineering Reference
In-Depth Information
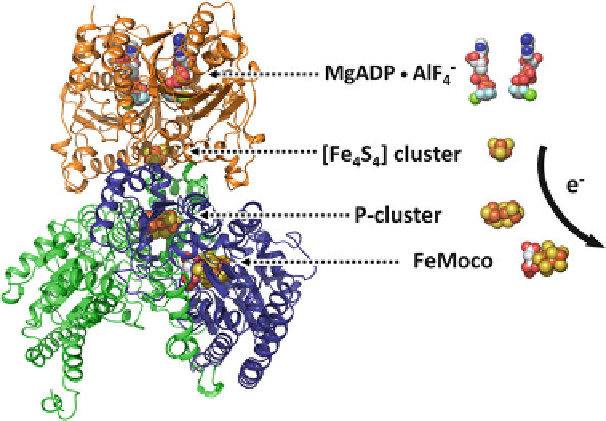
Figure 4 Crystal structure of half of the ADPAlF
4
-stabilized Fe protein/MoFe protein complex
(left) and the relative positions of components involved in the electron flow (right). The two subunits
of the Fe protein are colored orange, and the
-subunits of the MoFe protein are colored
blue and green, respectively. All clusters and ADP
AlF
4
are shown as space-filling models. Atoms
are colored as follows: Fe, orange; S, yellow; Mo, cyan; O, red; C, white; N, dark blue; Mg, green;
Al, beige; F, light blue. PYMOL was used to create the figure (PDB entry 1 M34).
ʱ
-and
ʲ
3.1 The Thorneley-Lowe Model
The classical scheme of N
2
reduction, known as the Thorneley-Lowe model, was
first introduced in the 1980s to summarize the kinetic data of Mo-nitrogenase [
28
].
It consists of a so-called Fe protein cycle and a so-called MoFe protein cycle, each
representing the series of events or reactions that occur on each protein component
for the successful completion of one catalytic cycle by Mo-nitrogenase. It should be
noted that, while the MoFe protein cycle describes events required for the complete
reduction of one N
2
molecule to two NH
3
molecules, the Fe protein cycle only
depicts events involved in the donation of one electron to the MoFe protein upon
the hydrolysis of two ATP molecules. Thus, based on the proposed stoichiometry of
N
2
reduction (see eqn.
1
above), the completion of one MoFe protein cycle requires
the completion of eight Fe protein cycles.
3.1.1 The Fe Protein Cycle
The Fe protein cycle starts with the reduction of Fe protein either by dithionite
in vitro
or ferredoxins
in vivo
, which renders its [Fe
4
S
4
] cluster in the +1 oxidation
state (Scheme
1
). Subsequently,
the reduced Fe protein binds two MgATP
Search WWH ::

Custom Search