Environmental Engineering Reference
In-Depth Information
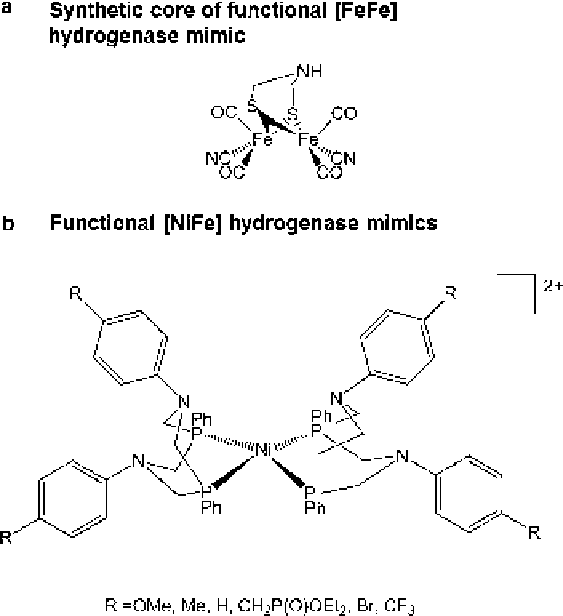
Figure 9 Structures of catalytically active synthetic small molecule hydrogenase mimics.
(a) derives from reference [
78
]; (b) derives from reference [
80
].
Historically [FeFe] hydrogenases have been the focus of synthetic analogue
studies [
74
], but highly active Ni enzyme analogues have been created recently
[
76
,
77
]. Studies on both systems have highlighted the mechanistic importance of
building a proton transfer site close to the H
2
-activating metal center in a H
2
catalyst
such as a hydrogenase. In the [FeFe] hydrogenases it has long been postulated that
the active site bridging dithiolate must be a dithiomethylamine rather than
containing a central bridging carbon or oxygen because it was proposed that the
nitrogen would play an essential role in holding a proton during catalysis. This
theory was finally proven in a recent synthetic analogue-based study [
78
]. Three
different [FeFe] enzyme active site mimics, possessing either a nitrogen- or carbon-
or oxygen-capped dithiolate ligand, were transported into a [4Fe4S] cluster
containing hydrogenase apo-protein and only the nitrogen-containing molecule
yielded a catalytically active H-cluster-containing enzyme (Figure
9
). Similarly,
in Ni model compounds the engineering of a second coordination sphere that can
function as a proton relay has dramatically accelerated the rate of H
2
evolution
(Figure
9
)[
79
,
80
].
Search WWH ::

Custom Search