Environmental Engineering Reference
In-Depth Information
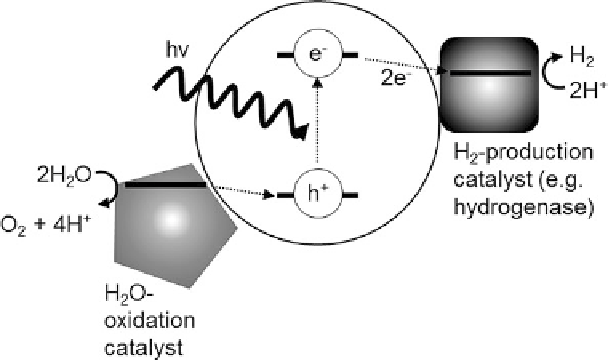
Figure 4 Schematic of molecular solar H
2
-production using a hydrogenase.
titanium dioxide (TiO
2
) electrode. The two electrodes were then placed into a
container of electrolyte solution, separated by a proton-exchange membrane. When
this photoelectrochemical cell was illuminated a photocurrent was induced as elec-
trons flowed from the porphyrin-TiO
2
anode to the hydrogenase cathode [
38
].
The advantage of using hydrogenase is that rapid H
2
production rates are reported;
for example, a H
2
-catalyst:organic-dye system was nearly 700 times faster when a
hydrogenase was used instead of a cobalt H
2
catalyst [
34
]. The problem with all of the
photo H
2
hydrogenase experiments conducted to date is that the oxidation reaction,
which completes the circuit by passing electrons to the photo-induced holes, has not
been water oxidation; true water splitting has therefore not been achieved. Instead
sacrificial electron donors have been used, such as reduced nicotinamide adenine
dinucleotide (NADH) in the porphyrin-TiO
2
cell. There are several reasons why
achieving photo-driven hydrogenase H
2
production
and
H
2
O oxidation is challenging
[
33
]. Hydrogenase-specific problems include the fact that the majority of hydroge-
nases are inactivated by O
2
; a large amount of protein encases the active site so the
maximum density of catalytic centers on a surface is low for a hydrogenase compared
to a nano-particulate catalyst; there is no well-established methodology for 'wiring'
hydrogenases onto surfaces [
33
]. To tackle these enzyme-related problems, solar H
2
devices incorporating hydrogenase-inspired synthetic analogues have also been
constructed [
33
], and hydrogenase-mimics are described in more detail in Section
5
.
Regardless of the nature of the H
2
catalyst, to achieve solar H
2
O-splitting the
photoexcitation process must generate both a photo-excited electron of electro-
chemical potential more negative than
E
(2H
+
/H
2
), and a photo-induced hole of
electrochemical potential more positive than
E
(O
2
/H
2
O). Finding light-capture
materials which both absorb light in a part of the spectrum that is not extensively
blocked by the ozone layer and generate electrons and holes of the right energy
to match water splitting is an ongoing challenge. To ensure an efficient rate of
solar energy capture the rate of water oxidation must be fast. Finding efficient
H
2
O-oxidation catalysts which are built from earth-abundant elements and stable
Search WWH ::

Custom Search