Biology Reference
In-Depth Information
Table 4.4 Alignment of AL with mitomiR 1974 from nuclear noncoding genome with indication
of the D-loop and A14 turn bend in yellow (Bandiera et al.
2011
; Demongeot et al.
2013a
,
b
,
c
;
Table 4.5 Consensus sequences of mito
2
miRs from mitochondrial noncoding genome (Sbisa
et al.
1997
; Cui et al.
2007
) and alignment with the Lewin ancestral tRNA D-loop and A14 turn
bend in yellow (
R ¼ puric¼¼AorG;Y¼ pyrimidic¼U, T, or C)
an enzyme that catalyzes the formation of deoxyribonucleotides used in DNA
synthesis from ribonucleotides, favoring the reduction of ATP and GTP, by
increasing dNTP pools and, hence, decreasing the NTP pools necessary for a
correct functioning of metabolisms like glycolysis.
A last but not least putative inhibitory mechanism comes from the regulation of
the tRNA function by hybridizing by RNA sequences, the tRNA loops especially
the tRNA D-loop: in Table
4.4
we see the possibility to hybridize tRNA loops
(especially the D-loop and the A14 turn responsible of the tRNA tertiary structure;
cf. Fig.
4.11
) by the nuclear mitomiR 1974 and in Table
4.5
, two possibilities of
small mitochondrial RNAs called mito
2
miRs [C 116, CSBD 353 in Sbisa
et al. (
1997
); Cui et al. (
2007
)] coming from the noncoding part of the mitochon-
drial genome, the Lewin's invariant part of tRNA secondary structure (Bandiera
et al.
2011
; Griffiths-Jones et al.
2005
) serving as reference template for tRNA
loops hybridization (Lewin et al.
2011
; Turner et al.
2005
; Sbisa et al.
1997
; Cui
et al.
2007
; Bandiera et al.
2012
). The unspecific inhibitory noise caused by this
possible direct (inside the mitochondrion matrix) new regulation favors as indicated
in the previous section the circuits with sufficiently strong interactions for resisting
to the mitomiR inhibitory influence (Demongeot and Waku
2012b
).
4.4.4 MicroRNas and Cellular Energetics: Glycolysis/
Oxidative Phosphorylation Coupling
MicroRNAs inhibit all glycolytic steps (cf. Figs.
4.12
and
4.13
, and Table
4.6
) and
depending on the intensity of these inhibitions favor at the level of pyruvate
coupling, the entrance in the Krebs cycle, if translocase and ATPase are not











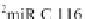
































Search WWH ::

Custom Search