Biology Reference
In-Depth Information
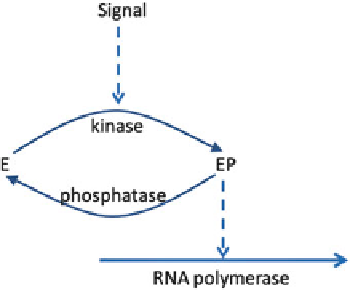
Fig. 3.3 A simple case of
signal transduction with a
single cascade of a kinase
and a phosphatase effecting
the phosphorylation of
transcription factor
E
, which
activates RNA polymerase
into transcription. The
external signal S activates the
kinase
control is the inverse of local properties, but highly significantly it is a matrix
inverse (inverse of the whole) rather than the collection of the individual inverses of
the local properties (Westerhoff and Kell
1987
; Kahn and Westerhoff
1991
).
3.7 Determination of Control Coefficients
The obvious way of determining control coefficients experimentally is to follow
their definition (see above), i.e. to perturb one step and let the system settle to a new
steady state and then measure the change in the variable of interest. There are many
ways of perturbing the rate of a reaction, each of them with its own advantages and
disadvantages:
1. Alteration of enzyme concentration by genetic means [e.g. Flint et al.
1981
;
Dykhuizen et al.
1987
; Fell
1992
; Niederberger et al.
1992
; Jensen et al.
1993
)
for further overview] has the disadvantage that other enzyme concentrations
may also change. This then invokes a second type of control coefficient
(Westerhoff and Workum
1990
).
2. Titration with inhibitors (e.g. Groen et al.
1982
) has the disadvantage that the
in situ efficacy of the inhibitor needs to be assessed.
3. Titration with purified enzyme in vitro (e.g. Torres et al.
1986
; Moreno-S´nchez
et al.
2008
) has the disadvantage that it only determines the control coefficient in
the in vitro situation.
The perturbation should be specific. Should one desire a complete picture of the
control of the variable in question, then the same procedure will have to be repeated
for each step of the system. In addition, the perturbations should be small—because
the steady state moves when the perturbations are finite, there is an error associated
with large perturbations. However, the effects of small perturbations are usually
also small and difficult to detect accurately. If one wants to use the enzyme
concentration as the parameter to perturb, the rate of reaction must change propor-
tionally with the enzyme concentration. If the relation between rate and enzyme
Search WWH ::

Custom Search