Biology Reference
In-Depth Information
a
b
100
200
180
80
160
140
60
120
100
40
80
60
Willamson et al., 1976
20
40
20
0
0
0.0
0.2
0.4
0.6
0.8
1.0
0.0
0.2
0.4
0.6
0.8
1.0
[ADP], mM
Relative workload
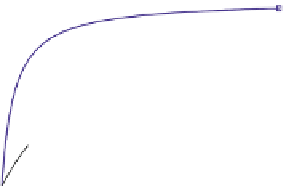
Fig. 11.11
The role of restriction of ADP diffusion in the regulation of mitochondrial respiration
.
(a) Kinetic analysis of ADP-activated respiration. The ADP concentrations corresponding to
mathematically modeled fluctuations of ADP by Michaelis-Menten graph representation with
colored small arrows (
black, red and green
), contained in the area of physiological cytosolic ADP
concentration (indicated by a
gray box
). When MOM is permeable, as in isolated mitochondria (
Δ
,
K
m
app
ADP—7.9
M), the regulation of respiration is impossible because of a saturated
ADP concentration for the minimal workload. When the ADP diffusion is restricted at the level of
MOM,
1.6
μ
(
circle
,
K
m
app
ADP—
as
in mitochondria
in
permeabilized
cardiomyocytes
370.75
M), the respiration rates become linearly dependant on ADP concentrations, in
fact also on heart workloads in accordance with the Frank-Starling law (b). This linear dependence
under physiological conditions can be amplified by creatine (see large
blue arrows
in a) in the
presence of activated MtCK (
Square
,
K
m
app
ADP—50.24
30.57
μ
M). Reproduced from (Guzun
et al.
2009
) with permission. (b) The metabolic aspect of the Frank-Starling's law of the heart is
expressed by linear dependence between the increase of left ventricular end-diastolic volume and
the increase of respiration rates in the absence of measurable changes in the intracellular ATP and
PCr content. Reproduced from (Saks et al.
2006c
) with permission
7.98
μ
Cr. When these conditions are fulfilled, activation of the coupled MtCK within MI
by Cr induces ADP/ATP recycling and increases respiration rate, thus amplifying
the effect of cytoplasmic ADP; under these conditions, the apparent
K
m
for ADP
becomes equal to 50.24
μ
M (Fig.
11.11a
). These data suggest that modula-
tion of respiration by local changes in ADP concentration, under condition of
restriction of adenine nucleotide diffusion across mitochondrial membranes, is
mediated by the structural organization of the MI. The MtCK reaction amplifies
the ADP signal due to its functional coupling with ATP Synthasome (Fig.
11.7
),
thus increasing the steady-state rate of adenine nucleotides cycling in mitochondria
and the rate of respiration. The coupled reactions of muscle type MM-CK in
myofibrils and MtCK in mitochondria perform under nonequilibrium conditions
and proceed in opposite directions (Fig.
11.10a-c
) (Saks et al.
2012
; Guzun
et al.
2009
; Guzun and Saks
2010
; Timohhina et al.
2009
). This mode of function
results in separation of energy fluxes (mass and energy transfer by PCr) and
signaling (information transfer by oscillations of cytosolic ADP concentrations,
Pi and PCr/Cr ratio) that is amplified within the MI. As a result, reactions catalyzed
by different isoforms of compartmentalized CK tend to maintain the intracellular
metabolic stability. The separation of energy and information transfer is illustrated
7.98

















































































































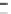








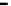
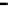
Search WWH ::

Custom Search