Biomedical Engineering Reference
In-Depth Information
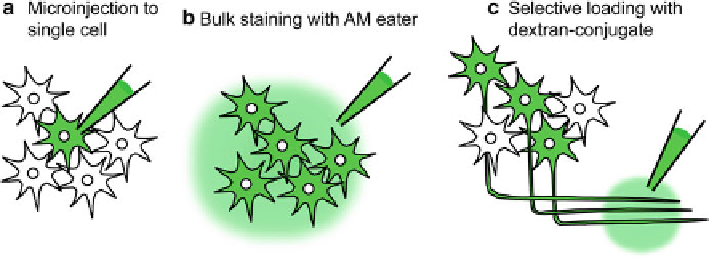
Fig. 5.2
Three classes of dye-loading methods. (
a
) For staining of single neuron, cell-impermeable
Ca
2+
indicator can be injected into the cell through a sharp or whole-cell patch electrode. (
b
) Bath
application of AM ester of the Ca
2+
indicator via a micropipette inserted into the nervous tissue is
used for bulk staining of many neurons. (
c
) Uptake of the dextran-conjugated Ca
2+
indicators into
the axonal fi bers running through a specifi c tract is capable of selective staining of projection
neurons
cell-permeable form of the indicator developed by Tsien (Tsien
1981
). The AM
ester of the indicator is, itself, insensitive to Ca
2+
and will not fl uoresce outside of
cells. Once inside the cell, however, the AM ester of the Ca
2+
indicator is hydrolyzed
by intracellular esterase, restoring the Ca
2+
sensitivity and also making the indicator
membrane impermeable so that it will not leak out. For staining cells in culture or
in brain-slice preparations, the AM ester of the Ca
2+
indicator is delivered along
with a nonionic detergent, Pluronic F-127 or PowerLoad (Invitrogen), which are
reagents that help disperse the indicator (Fig.
5.2b
). To use the AM ester staining
techniques to in vivo preparations, the loading solution is typically pressure injected
directly into the brain or ganglion preparation (Garaschuk et al.
2006
). However,
there is considerable variability in the loading effi ciency between different neuronal
tissues, between different cell types within a single tissue sample, and between dif-
ferent animal species. This variability is due to differences in membrane permeabil-
ity for the AM ester and in the endogenous intracellular esterase activity. Since the
AM ester is loaded not only into neurons but also into glial cells, dual staining with
Ca
2+
indicator and astrocyte markers is often required to distinguish neuronal
responses from Ca
2+
signals originating from the glial cells (Garaschuk et al.
2006
).
Dextran-conjugated Ca
2+
indicators are useful for selective loading into axonal
fi bers that lie within specifi c nerve tracts. Dextrans are hydrophilic polysaccharides
characterized by their high molecular weight, high water solubility, and low toxic-
ity. Due to these properties, fl uorescent dextrans have been used for anterograde and
retrograde neuron tracing. Dextran-conjugated dyes loaded into the neurons are
effectively transported by axonal traffi cking and allow staining of a specifi c group
of neurons (Fig.
5.2c
). For example, a highly concentrated chip of dextran-
conjugated Ca
2+
indicator can be placed on a specifi c location within the brain and
will be taken up by a very restricted set of axons in contact with that chip (Gelperin
and Flores
1997
; Delaney et al.
2001
; Sachse and Galizia
2002
).