Biomedical Engineering Reference
In-Depth Information
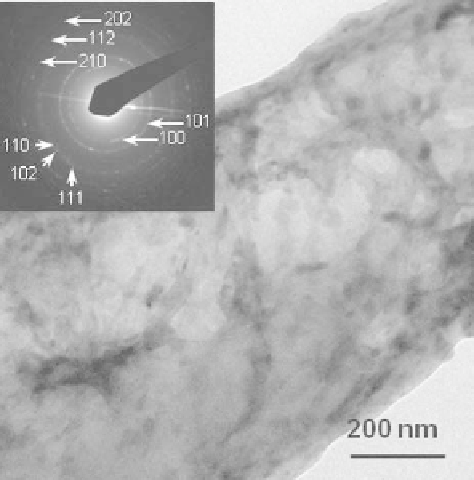
Figure 12.12
TEM images of CNXL-supported seleniumnanoparticles prepared at 160
◦
C:
An inset denotes the SAEDpattern (reprintedwithpermission fromref 61). Copyright 2007
Elsevier.
of MB. 94.7% of the initial MB was degraded within 1.5 hours and no more MB was
detected after 4 h photoreaction. The Se/UV photocatalytic degradation of MB in an
open reactor resulted in an almost complete mineralization of carbon and of nitrogen
and sulfur heteroatoms into CO
2
,NH
4
,NO
3
and SO
2
4
, respectively as seen below
(Equation 12.4) (64).
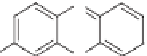
Methyleneblue(MB)
N
+
−
Methylene blue (MB)
(CH
3
)
2
N
S
N(CH
3
)
2
Cl
(12.4)
2MB
+
25O
2
→
2HCl
+
2H
2
SO
4
+
6HNO
3
+
32CO
2
+
12H
2
O
12.5
Conclusion
This chapter demonstrated the application of green chemistry principles in the synthe-
sis of metallic nanoparticles on CNXL without additional reducing agents, as well as
the preparation of porous metal oxides and metal carbide nanorods. Thermal treat-
ment is necessary to initiate the reduction of metal ions by the electron donation of
CNXL. Surface hydroxyl groups anchor metal ions initially deposited and reduces metal
ions without major collapse of CNXL structure upon thermal treatment for the metal
ions with high reduction potentials such as Ag(I), Au(III), Pt(IV), Pd(II), and Se(IV).

Search WWH ::

Custom Search