Biomedical Engineering Reference
In-Depth Information
of metal ion solutions to CNXL suspension resulted in loosely packed precipitates,
which were separated from the liquid by centrifugation. The CNXL films with metal
ions incorporated were dried at room temperature in air. CNXL itself showed some
surface oxidation, which had a FT-IR carbonyl stretching band at 1720 cm
−
1
,which
was generated during the hydrolytic preparation. After adding metal ions to the CNXL,
the carbonyl band was decreased due to the interaction between the carboxyl groups and
metal ions. Thermal treatment at 160-200
◦
C initiates the redox reaction on the composite
film, and the metal ions are reduced by the CNXL surface while some hydroxyl groups
on the CNXL were oxidized into carboxyl group again, which can be seen from the
reappearance of the peak around 1720 cm
−
1
(50).
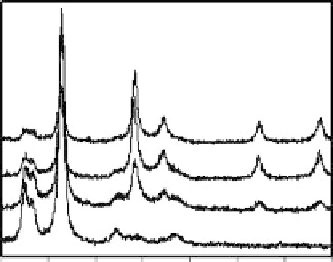
Figure 12.8 shows XRD traces of reduced Ag nanoparticles on the CNXL. As-prepared
Ag(I)/CNXL composite film does not contain any crystalline silver even after several
days at room temperature, indicating that room light is not enough to reduce silver (I)
ions at the temperature. The composite film thermally treated at
150
◦
C did not show
any crystalline silver peaks because the elemental silver reduced by CNXL surface did
not nucleate to grow into nanocrystals, but silver nanocrystals were big enough to be
observed at 160
◦
C. After the low Ag loading samples (3.1 wt%) were treated at 200
◦
C
for 2 h, atomic silver was observed, reflected by the diffraction peaks on the XRD
pattern assigned to silver crystals at 2
θ
=
38
.
35, 44.70, 64.60, and 77
.
60
◦
for (111),
(200), (220), and (311) (Figure 12.8). At higher loadings (10.7 and 17.5 wt%), the
diffraction peaks were much stronger and sharper, indicating that the Ag particles were
bigger (Figure 12.8c and d). The diffraction peaks of CNXL, although slightly collapsed,
have been observed for all the samples. This process (at 200
◦
C in air) does not collapse
the crystallinity of the CNXL and reduce silver ions to silver nanoparticles.
Figure 12.9 reveals microscopic images of metal/CNXL composites treated at 200
◦
C
for 2 h in air. Low loadings (3.1 wt%) of silver clearly retain the structure and mor-
phology of the original CNXL (not shown here). The sparsely dispersed particles were
5-10 nm in size. However, it is hard to observe silver metal on the surface by FESEM.
There are a large number of silver particles on the CNXL surface in case of large silver
(d)
(c)
(b)
(a)
10
20
30
40
50
60
70
80
2
q
Figure 12.8
XRD traces of Ag/CNXL composites as function of Ag loading after thermal
treatmentat200
◦
Cfor2h; (a)3.1wt%as-prepared,(b)3.1, (c)10.7,and(d)17.5wt%.
Search WWH ::

Custom Search