Biomedical Engineering Reference
In-Depth Information
TEOS/H
+
1400
°
C/Ar
Amorphous region
Crystalline region
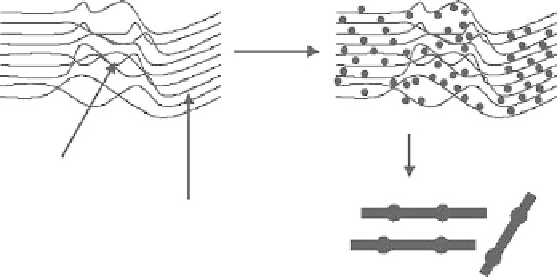
Figure 12.5
A proposed mechanism for the formation of SiC materials with camelback
morphologies frombleached pulp (with kind permission fromSpringer Science
+
Business
Media:ref30).Copyright2007Springer.
approaches for the preparation of advanced materials are desirable. For example, sev-
eral carbohydrate materials including glucose (33), chitosan (34), cellulose (35), polyol
(36), and pollen grains (37) have recently been used to prepare metal nanoparticles as a
'green' synthetic process.
Among the transition metal nanocrystals, nickel and copper nanocrystals are diffi-
cult to prepare due to their highly oxidizing property. The magnetic and mechanical
property of nanostructured nickel and copper has attracted much attention especially in
fuel cell and catalytic applications (38, 39). To date, many different morphologies of
metal nanocrystals such as nanowires (40), nanotubes (41), and nanorods (39) have been
prepared via various approaches including hydrothermal reduction (42), chemical vapor
deposition (43), and electrodeposition (44). For the solution processes, the decomposi-
tion of organometallic nickel precursors such as M(CO)
4
,M(Cp)
2
, and M(COD) (M
Ni
or Cu) in organic solvents are required (45). Reducing and stabilizing agents, including
surfactant molecules, are also critical for this synthesis.
A simple and green method for the preparation of transition metal nanoparticles using
a CNXL suspension by
in-situ
thermal reduction without additional reducing agents
is introduced in this section (46).
−
The carbonized CNXL serves to stabilize metal
nanocrystals.
The rodlike CNXLs can be easily observed in as-prepared samples, indicating that
metal ions are stabilized on the surface of CNXL. Figure 12.6 shows the XRD patterns
of Ni products obtained after thermally treated at 200, 300, 400, and 500
◦
Cfor6h
under N
2
, respectively. At 200
◦
C, all the diffraction peaks of the CNXL were com-
pletely retained as mentioned earlier. The diffraction peaks at 2
θ
=
14
.
87, 16.59, 22.77,
and 34
.
32
◦
are for (110), (110), (200), and (004), indicating a typical cellulose I (25).
At 300
◦
C, the crystallinity of the CNXL was completely destroyed and no metallic
nickel peaks were observed.
However, at 400
◦
C, a very small diffraction peak (111)
at 2
θ
=
44
.
30 starts to grow. This peak is stronger and sharper with the elevation of
temperature and associated with other diffraction peaks at 2
θ
=
51
.
84, and 74.83, indi-
cating that all the reflections of the XRD pattern can be finely indexed to a face-centered
Search WWH ::

Custom Search