Biomedical Engineering Reference
In-Depth Information
1.0
0.8
0.6
0.4
0.2
1000
10,000
M (g mol
−
1
)
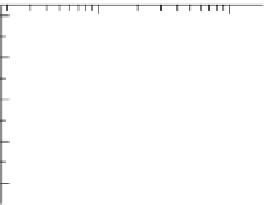
Figure7.2
Phenolicgroupsperstructuralunit(SU)versusmolecularmassforsoftwoodkraft
lignin. The full curve represents the outcome of the computer calculated Guarana model
(Jurasek 1995). The broken curve is a fit to the values calculated from conductometric
titration(
),are
calculatedbased on the corresponding phenolic content given by the full curve but taking
intoaccountthemolecularmassdistributionsofthesamplesrepresentedbytheemptycircles.
ReproducedwithpermissionfromNorgrenandLindstrom(2000a).Copyright (2000),Walter
deGruyter.
◦
)andthenumber-averagemolecularweight.Thedatagivenbyfilledcircles(
•
dissociation. Since the number of counterions of a polyelectrolyte generally is large, the
latter is often most important to consider and it explains why polyelectrolytes usually
are more soluble than uncharged polymers (J onsson
et al
. 1998).
After depolymerising the lignin in the fibre wall the KL is solubilised mainly through
dissociation of phenolic groups, due to the alkaline conditions in the digester. The KL
fragments formed are widely polydisperse, both chemically and physically (see Figure 7.2).
For example, generally high molecular weight KL fragments might be considered having
their pKa's at much higher levels than low molecular species (Norgren and Lindstrom
2000b). For polyelectrolytes carrying weakly acidic groups, the dissociation and thus the
solubility is governed by an increase in hydroxide ion concentration. This is also the case
for KL:s. The pK
a
value of coniferyl alcohol, the most frequent structural unit in softwood
lignin, is 10.25 at room temperature, as calculated from Hammet equation (Perrin 1981).
When the temperature is elevated in a system containing neutral electrolytes, the
solubility of the salt increases due to the increased entropy. This is often also valid
for polyelectrolytes in aqueous solutions. However, concerning polyelectrolytes bearing
weakly acidic groups, the explanation is not as straightforward as it may seem. In
Table 7.3, data of the dissociation behaviour at different temperatures of some phenolic
substances are presented.
For all substances investigated the pK
a
values decrease as the temperature increases,
normally indicating increased dissociation. At the same time, the negative logarithm of
the ion product constant of water, pK
w
decreases even more, see Figure 7.3.
Due to
that, the net dissociation (
α
) will decrease when the temperature is elevated.
The polydispersity of KL will of course also introduce differences in the solubility and
colloidal stability characteristics within the macromolecular distribution of fragments, see
Figure 7.4.
Numerous studies dealing with the colloidal behaviour of lignin derivatives have earlier
been presented in the literature (Junker 1941, Lindstr om 1980, Sarkanen
et al
.
1982,












Search WWH ::

Custom Search