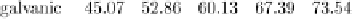Graphics Reference
In-Depth Information
Figure
.
.
Muscle data: distribution statistics and boxplots for adjusted weights (Files:
hh/dsgn/code/jsm.cc
.s, hh/dsgn/code/jsm.cc
.s)
Example - Muscle Data, continued
6.7.5
he Muscle data was introduced in Sect.
.
.
. In Fig.
.
we display microplots that
compare the distributions of responses for each level of the factor
current
.he
microplots in Fig.
.
are horizontally oriented. hese consume even less vertical
pagespacethantheverticallyorientedboxplotsinFig.
.
.headvantageofreduced
pagespacewithahorizontal orientation mustbeweighedagainst apreferenceforthe
vertical response scale for vertically oriented boxplots.
Inthissetting, weareabletoalignthenumberswithasimilarinterpretation. hus,
forexample, all the means are in the same column. Vertical alignment of comparable
numbers oten makes it easier for the reader to understand them.
Graphical Display of Incidence
and Relative Risk
6.8
In a clinical trial of any new pharmaceutical product, any adverse events (negative
sideeffectsofthetreatment) mustbereportedbythecompany sponsoringthetrial to
the US Food and DrugAdministration. hese data in file (h
/datasets/aedotplot.dat)
from Amit et al. (
) is based on a clinical trial at GlaxoSmithKline.
Figure
.
compares the incidence rate of multiple adverse events in a clinical
trial. Itisintended as agraphical summary of adverse events inthe trial, highlighting
the key differences between two treatments. It is a panel plot with two associated
dotplots. he let-hand dotplot gives the comparative incidence of selected adverse
events under each of the two treatments. he right-hand dotplot gives estimates and
isolated
% confidence intervals for the associated relative risks.
We place a vertical reference line at relative risk=
to facilitate the interpretation
that an event having aconfidence interval including
doesnot distinguish the effects
of treatments A and B. Events having confidence intervals that do not cross
suggest
a significant difference between treatments A and B.
he adverse events are ordered by relative risk, so that those with the largest in-
creases in risk forthe experimental treatment are prominent at the top of the display.