Chemistry Reference
In-Depth Information
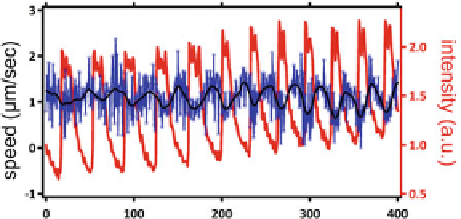
Fig. 6.9
The speed as a func-
tion of time for the squirmers
when an oscillating chemical
reaction (B-Z) takes place
in the droplet. The optical
transmission of the droplet is
plotted in red (arbitrary units,
linear scale) along with the
velocity trace
as described in the previous chapter. This oscillation can be easily visualized by
the optical transmission of the droplet. Care was taken that there were no spatio-
temporal patterns within the droplets, in contrast to some other recent work on droplet
locomotion [
31
]. Figure
6.9
shows an overlay of the the B-Z chemical oscillations
and the speed of the swimming droplet, which are anti-phase with each other. As it is
known for the B-Z reaction, the decrease of the transmitted intensity corresponds to
an increase in the bromine concentration within the droplet. It is obviously possible
to control the droplet velocity in some range, and we have demonstrated here how
this can be done even in an autonomous manner.
Finally, we come to the discussion of directionality, which together with the
speed characterises the squirmer velocity. In Fig.
6.3
, we see that some of swimmer
tracks are 'smoother' in appearance than others. In other words, some swimmers
seem more persistant in their direction than others. However, though the tracks are
displayed together, they correspond to swimmers at various times after the initial
droplet formation. For a given swimmer, we calculate its directionality, defined as
is the turn angle i.e. the angle between the velocity vectors of
the swimmer at equidistant time points, as a function of time. As seen in the top
panel of Fig.
6.10
, which shows the data for surfactant concentration of 100 mM, the
directionality increases linearlywith time and after
cos
φ
, where
φ
∼
200 s, it reaches a plateau around
∼
4. As seen in the lower panel of
6.10
, this remains roughly constant for a range of
surfactant concentrations. Therefore, the change in the directionality of the droplet
is likely to be a consequence of the driving from within, namely the bromine source.
Initially, when there is a surplus of bromine, the droplets gets 'kicked' around due to
the rapidly changing interface conditions. However, as this rate reduces, a balance is
reached between the reaction from inside and the replishment of the surfactant from
the outside. At this stage, the surface coverage is maintained around an equilibrium
value, thus making the motion homogeneous and directionally persistant.
0
.
6.4 Summary and Outlook
The Belousov-Zhabotinsky reaction running inside micrometric droplets with mono-
olein as a surfactant renders them active: capable of chemical communication as
we showed in the previous chapter and also capable of locomotion as described in