Biology Reference
In-Depth Information
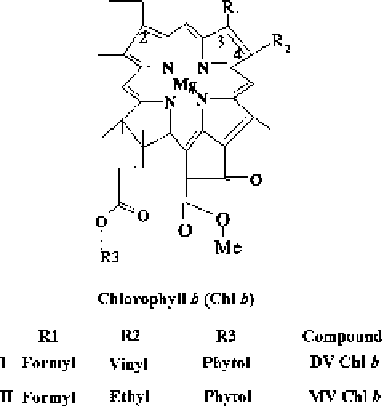
Fig. 2.12 DV and MV
Chl
b
2.10.2 Discovery of Novel Chl Biosynthetic Routes
Chlorophyll biosynthetic heterogeneity refers either (a) to spatial biosynthetic
heterogeneity, (b) to chemical biosynthetic heterogeneity, or (c) to a combination
of spatial and chemical biosynthetic heterogeneities (Rebeiz
2010
). Spatial biosyn-
thetic heterogeneity refers to the biosynthesis of an anabolic tetrapyrrole or end
product by identical sets of enzymes, at several different locations of the thylakoid
membranes. On the other hand, chemical biosynthetic heterogeneity refers to the
biosynthesis of an anabolic tetrapyrrole or end product at several different locations
of the thylakoid membranes, via different biosynthetic routes, each involving at
least one different enzyme.
The chemical heterogeneity of Chl resides mainly in the MV or DV substitutions
at positions 2 and 4 of the Chl macrocycle (Figs.
2.7
, 4c, 4d and
2.12
). It also
involves esterification with different long chain fatty alcohols of the propionic acid
residue at position 7 of the macrocycle, and substitution of a lactone ring for a
cyclopentanone ring at positions 5 and 6 of the macrocycle (Wu and Rebeiz
1988
).
This chemical heterogeneity is catalyzed by various Chl biosynthetic routes and
involves various enzymes. One family of enzymes, the vinyl reductase enzyme
family plays a prominent role in this process. All these issues will be discussed in
reductases is given below.
2.10.2.1 Discovery of DV Mg-Proto Vinyl Reductase
4-vinyl Mg-protoporphyrin IX reductase (VMPR) catalyzes the reduction of the
vinyl group of Mg-Proto to ethyl, at position 4 of the Macrocycle (Fig.
2.7
, 2a, 2b).
Search WWH ::

Custom Search