Biology Reference
In-Depth Information
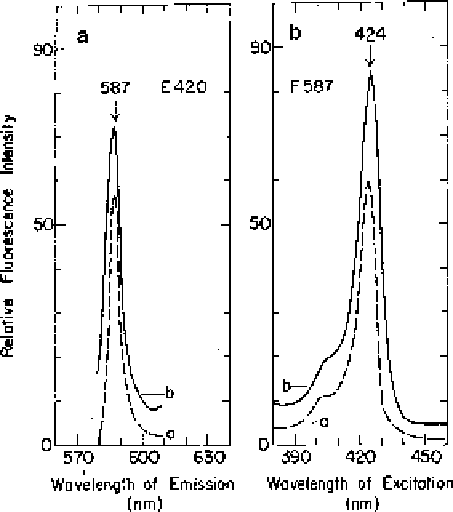
Fig. 18.3 Fluorescence
spectra of Zn-Proto in ether
at 77 K. (a) Fluorescence
emission and (b)
fluorescence excitation
spectra in ether at 77 K
of (
a
) authentic Zn-Proto
and (
b
) of the ether extract
of third instar
T
.
ni
larvae.
The larvae were sprayed
with 40 mM ALA + 30 mM
Oph and were incubated in
darkness for 17 h prior to
extraction. The emission
and excitation spectra were
recorded at the emission (
F
)
and excitation (
E
)
wavelengths indicated on
the figure, at 4 nm emission
and excitation slit widths.
Arrows
point to
wavelengths of interest
(Adapted from Rebeiz
et al.
1990a
)
c
oxidase, and under normal conditions are only released when they are fully reduced
to H
2
O (Halliwell
1984
). It is conceivable therefore that premature release of these
radicals in the intracellular environment may trigger peroxidation of the membrane
lipoprotein, causing the same type of damage as singlet oxygen-mediated photody-
namic damage. This explanation is compatible with the observed accumulation of
Zn-Proto in treated insects. Indeed, Zn-Proto is not a natural metabolic intermediate
of the porphyrin-heme pathway. Its occurrence in living cells and tissues usually
denotes a poisoned porphyrin-heme metabolism (Lamola and Yamane
1974
). Most
ferrochelatases (the enzymes that insert ferrous iron into Proto to form heme) can
insert Zn instead of iron into Proto to yield Zn-Proto, particularly under unfavorable
reaction conditions (Lamola and Yamane
1974
). Thus it is possible that the accu-
mulation of Zn-Proto as a result of treatments containing Dpy or Oph may be caused
by damage to the ferrochelatase system causing the enzyme to insert Zn instead
of ferrous Fe into some of the Proto. If it ensues that some of the cytochrome
c
prosthetic groups consist of Zn-Proto instead of heme in treated insects, then
those cytochrome
c
oxidase molecules containing Zn-Proto instead of heme may no
longer be able to prevent the premature release of oxygen superoxide and hydroxy
free radicals, by holding them tight to the reaction centers until they are fully
reduced. The intracellular release of these destructive free radicals in the biological
membrane environment could then contribute to the free radical damage that results
in insect death.
Search WWH ::

Custom Search