Biology Reference
In-Depth Information
5.4 Biosynthesis of Coproporphyrinogen III (Coprogen III)
Coprogen III is the precursor of protoporphyrinogen IX. It is formed from
Urogen III by decarboxylation, a reaction catalyzed by Urogen decarboxylase
which converts Urogen III to Coprogen III (Granick and Mauzerall
1958
;
Mauzerall and Granick
1958
). Stepwise decarboxylation of the 4 acetate side
chains and the resulting structures of the intermediates led to the proposal that the
acetate side chains on rings D, A, B. and C are decarboxylated in a clockwise
fashion starting with ring D (Jackson et al.
1976
,
1980
). Although this appears
to be the case in patients suffering from porphyria cutanea tarda, a random
rather than an ordered decarboxylation appears to prevail in normal individuals
(Luo and Lim
1993
). These observations led to the proposal that the substrate
binding site has such a flexible architecture that at low Urogen concentrations,
decarboxylation may be ordered, while at high substrate concentrations it may be
random (Akhtar
1994
). The DNA coding for Urogen III decarboxylase in humans
(Romeo Romeo et al.
1986
) and rats (Romana et al.
1987
) has been cloned and
sequenced. The human enzyme consists of 367 amino acids with a molecular
weight of 40,831. In animal cells Coprogen III is formed in the cytoplasm
(Rebeiz et al.
1996
)). In plants Urogen III decarboxylase appears to be loosely
bound to the plastid membranes (Lee et al.
1991
). Beyond the possibility that in
higher plants Coprogen III may contribute to the formation of Proto in five
different environments (Table
5.1
), no specific efforts have been made to docu-
ment the nature and extent of Coprogen III biosynthetic heterogeneity in plants
(Figs.
5.10
and
5.11
).
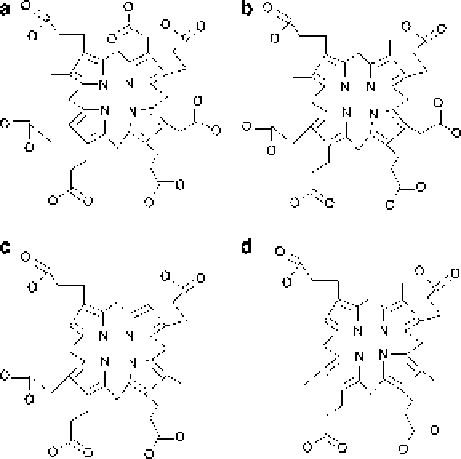
Fig. 5.10 (a)
Heptaporphyrinogen III, (b)
hexaporphyrinogen III, (c)
pentaporphyrinogen III and
(d) coproporphyrinogen III
Search WWH ::

Custom Search