Environmental Engineering Reference
In-Depth Information
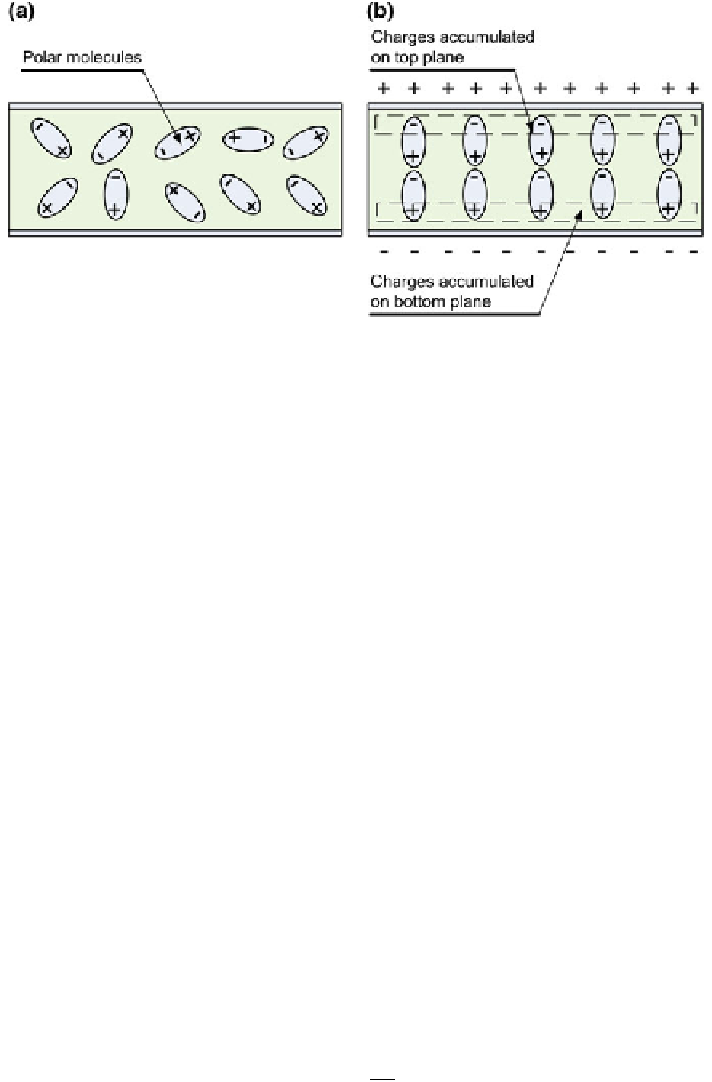
Fig. 10.1 a Schematic presentation of the orientation of polar molecules in a dielectric material
that is not exposed to an electric eld, b Schematic presentation of the orientation of polar
molecules in a dielectric material under the in
fl
uence of an electric eld
Another consequence of a transition from the disordered to the ordered state is a
reduction of the material
'
s dipolar entropy. If the dielectric material is subjected to a
rapid change of electric
eld, so that the heat transfer to the surroundings can be
neglected, the process can be considered as adiabatic. In this case the total entropy of
the material must remain constant, which then causes the temperature of the elect-
rocaloric material to increase. When the electric
eld is turned off, the electrocaloric
material transits back to the disordered phase and its temperature decreases. If the
polarization is performed under isothermal conditions, the dipolar entropy change of
the electrocaloric material will lead to an increase in the total entropy of the elect-
rocaloric material (or a decrease in the total entropy due to the depolarization).
The electrocaloric effect is analogous to the magnetocaloric effect. However,
instead of a magnetic
eld change, the electrocaloric effect is induced by an electric
eld change. If the entropy of the dielectric material is considered as a function of
the temperature and the electric
eld, then the total change of the entropy can be
expressed as [
2
]:
s
s
Þ¼
o
o
ds
ð
T
;
E
dT
þ
dE
ð
10
:
1
Þ
o
T
o
E
E
T
where s and T are the speci
c entropy and the temperature of the material,
respectively, and E is the electric
eld. Next, the speci
c heat at constant electric
eld c
E
and the speci
c heat at constant temperature c
T
are de
ned using the
following relations:
s
T
o
c
E
¼
ð
10
:
2
Þ
T
o
E

Search WWH ::

Custom Search