Biomedical Engineering Reference
In-Depth Information
the basis of its highly cationic density suitable for DNA condensation. CDs,
instead, represent a class of cyclic oligosaccharides with six to eight -glucose
units with intriguing hydrophobic cavities. Upon inclusion of β-CD with CHIT,
adamantane-modiied pyrene (ADA-PY)
(Fig. 5.10a,b)
and MWCNTs (Fig.
5.10c), the synergistic effect between aromatic residues (especially in pyrene
molecules PY) and cationic groups (mainly in CHIT) greatly improved CHIT's
DNA-condensing eficiency.
As shown in Fig. 5.10,
β
-CDs preferred accommodating the cage of
adamantane inside their cavity, while exposing pyrene grafts outside. In the
presence of such assemblies, DNA chains were condensed to uniform hollow
loops, as shown in Fig. 5.11, prepared using AFM.
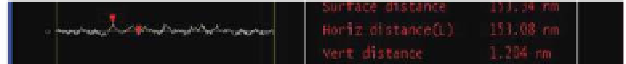
suface distance 153.34 nm
Horiz distance(L) 151.08 nm
Vert distance
1.204 nm
Angle
0.451°
Figure 5.11
AFM images of DNA in the presence of the supramolecular assemblies.
Reproduced from Liu
et al.
37
with permission. See also Colour Insert.
The exploitation of multifunctional structures for their use as
electroanalytical devices induced Shie
et al.
to combine MWCNTs with both
DNA and cytochrome
c
protein (cyt
c
), which has shown a unique binding
property with protein redox partners.
38
The formation of a biocomposite ilm
among MWCNTs, DNA and cyt
c
consisted on the initial attachment (either
covalent, between the amino groups of DNA and the activated carboxylated
functions on CNTs, or non-covalent, by simple hydrophobic interaction of
DNA with the tubes' side walls), and subsequent attachment of cyt
c
on the
DNA.
39
This second step was based on the electrostatic interaction between
the positively charged groups (e.g., lysine-NH
3
+
, or Fe
2+
and Fe
3+
in cyt
c
) and







Search WWH ::

Custom Search