Biomedical Engineering Reference
In-Depth Information
1) ( CH
2
O)
n
, DMF
+
Cl
-
O
NH
3
O
O
NH Bo c
N
HO O C
N
H
O
120°C, 3 days
2) HC l
1
2
1 ) Bo c-L ys (B o c)- Gly- Ty r(t- bu t)-T yr (t-b u t)-Gly -OH
HATU, D IEA, DMF, 2 hours
2) TFA, 2.5 hours
O
N H -C O -G ly -T yr-T y r-G ly -L ys
O
N
3
Scheme 4.1
Functionalisation of CNTs via fragment condensation.
Another characterisation became feasible after the incorporation of
N
15
-labelled Gly at the C-terminal part of the sequence, which allowed for a
homonuclear and heteronuclear two-dimensional (2D) NMR analysis to be
performed. A broad correlation peak in the decoupled
15
N-
1
H spectrum of the
fully protected compound
3
, showing a maximum peak height at 119.6/7.40
ppm, was indicative of a uniform distribution of the peptide around the
nanotubes' sidewall. A series of bi-dimensional experiments then permitted
all the resonances of the peptide moiety to be assigned (Fig. 4.2). A decrease
in and a broadening of the signal intensities were observed for the amino acid
residues approaching the aromatic tube walls. All the predictable sequential
RH
i
-NH
i
+1
cross peaks were conirmed in the ROESY spectrum. Moreover, a
spatial correlation between the RH of glycine at position 5 and the amide
proton of the triethylene glycol chain conirmed the presence of a covalent
bond between the peptide and the CNTs (Fig. 4.2b).
Figure 4.2
Partial (a) TOCSY and (b) ROESY
1
H NMR spectra of peptide-CNT
3
in
H
2
O/
t
-BuOH-
d
9
(9:1) solution. Peptide residues are numbered from Lys1 to Gly5. TEG
denotes triethylene glycol. The TOCSY spectrum was recorded while decoupling the
15
N heteronucleus. Reproduced from Pantarotto
et al.
5
with permission.















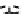

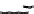




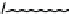

































































































































































Search WWH ::

Custom Search