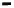Biomedical Engineering Reference
In-Depth Information
7
20
6
15
5
4
10
3
5
2
1
0
2
2.1
2.2
2.3
2.4
2.5
2.6
2.7
0
1.9
2
2.1
2.2
2.3
2.4
C
Fractal dimension,
D
f
D
Fractal dimension,
D
fd
5
2.7
2.6
4
2.5
3
2.4
2.3
2
2.2
1
2.1
2
0
0
20
40
60
80
100
120
140
0.9
1.0
1
1.1
1.1
1.2
1.2
1.3
E
F
Thrombin concentration (nM)
D
f
/
D
fd
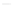
Figure 15.10—cont'd
(c) Increase in the binding rate coefficient, k for a single-fractal analysis with an increase in the
fractal dimension, D
f
. (d) Increase in the dissociation rate coefficient, k
d
for a single-fractal analysis
with an increase in the fractal dimension, D
fd
. (e) Increase in the fractal dimension, D
f
with an
increase in the thrombin concentration (in nM) in solution. (f) Increase in the affinity, K (
¼
k/k
d
)
with an increase in the fractal dimension ratio (D
f
/D
fd
).
The fit is very good. Only four data points are available. The availability of more data points
would lead to a more reliable and better fit. The binding rate coefficient
k
for a single-fractal
analysis is extremely sensitive to the fractal dimension
D
f
or the degree of heterogeneity that
exists on the SA chip surface as it exhibits an order of dependence between eleven and
twelve (equal to 11.62) on the fractal dimension
D
f
.
Figure 15.10d
and
Table 15.6
show the increase in the dissociation rate coefficient
k
d
with an
increase in the fractal dimension
D
fd
. For the data shown in
Figure 15.10d
, the dissociation
rate coefficient
k
d
is given by:
D
7
:
56
3
:
99
k
d
¼ð
0
:
0061
0
:
0048
Þ
ð
15
:
5d
Þ
fd