Biomedical Engineering Reference
In-Depth Information
2
1.4
1.2
1.5
1
0.8
1
0.6
0.4
0.5
0.2
0
0
0
0.2
0.4 0.6 0.8
Rabbit IgG concentration (
1
1.2
1.4
0.6
0.8 1 1.2 1.4 1.6
Dissociation fractal dimension,
D
fd
1.8
2
2.2
B
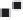
Figure 13.9
(a) Increase in the dissociation rate coefficient, k
d
with an increase in the rabbit IgG concentration (in
m
A
µ
m)
m) (b) Increase in the dissociation rate coefficient, k
d
with an increase in the fractal dimension, D
fd
.
Figure 13.9b
and
Tables 13.6 and 13.7
show the increase in the dissociation rate coefficient
k
d
with an increase in the fractal dimension for dissociation
D
fd
. For the data shown in
Figure 13.9b
, the dissociation rate coefficient
k
d
is given by:
D
1
:
94
1
:
38
fd
k
d
¼ð
:
þ
:
Þ
ð
:
Þ
0
295
1
038
13
6b
The fit is not good. There is scatter in the data. The availability of more data points would
lead to a more reliable fit. The dissociation rate coefficient
k
d
is sensitive to the degree of
heterogeneity that exists on the sensing surface during the dissociation phase
D
fd
as noted
by the close to second (equal to 1.94) order of dependence exhibited.
13.4 Conclusions
A fractal analysis is presented for the binding and dissociation (if required) of different
analytes on different biosensor surfaces. Both a single- and a dual-fractal analysis
were used. The dual-fractal analysis was used only when the single-fractal analysis did
not provide an adequate fit. This was judged using Corel Quattro Pro 8.0 (Corel Quattro
Pro, 1997) to see if the regression provided was adequate (regression coefficient greater
than 0.95).
A wide variety of examples available in the literature were analyzed. The systems analyzed
were selected at random. The analyte-receptor systems analyzed include (a) the binding of
ODN-P and noncomplementary ODN during the hybridization assay with EST2-A34 reporter
(
Wang et al., 2007
), (b) binding during the primer elongation reaction of DNA coupled