Biomedical Engineering Reference
In-Depth Information
2.5
1.7
1.6
1.5
1.4
1.3
1.2
1.1
1
0.9
650
2
1.5
1
0.5
0
0.9
1
1.5
1.6
1.1 1.2
Fractal dimension,
D
f
1.3
1.4
700
750 800
Temperature (K)
850
900
A
B
5
4
3
2
1
0
650
700
750 800
Temperature (K)
850
900
C
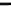
Figure 10.5
(a) Increase in the binding rate coefficient, k, with an increase in the fractal dimension, D
f
.
(b) Decrease in the fractal dimension, D
f
, with an increase in the temperature, T (in
K).
(c) Decrease in the fractal dimension for dissociation, D
fd
, with an increase in the temperature,
T (in
K).
Þð
Tin
K
Þ
28
:
59
D
fd
¼ð
2
:
9
10
81
2
:
1
10
81
ð
10
:
6e
Þ
The fit is reasonable. Only five data points are available. The availability of more data points
would lead to a more reliable fit. The fractal dimension in the dissociation phase,
D
fd
, is extremely
sensitive to the temperature as it exhibits a negative 28.59 order of dependence on the temperature
in the 673-873
K range. No explanation is offered, at present, to elucidate this extremely high
order of dependence exhibited by the fractal dimension in the dissociation phase,
D
fd
,onthetem-
perature,
T
(in
K) in the 673-873
K range. It is of interest to note that
D
f
as well as
D
fd
exhibit
decreases as the temperature increases. In other words, the degree of heterogeneity on the biosen-
sor surface, in this case, decreases as the temperature increases in the 673-873
K range.
Roy et al. (2005)
also analyzed the influence of NH
3
concentration in ppm in air on its bind-
ing and dissociation kinetics to the sol-gel derived thin film biosensor.
Figure 10.6a
shows
the binding and dissociation of 160 ppm NH
3
in air to the sol-gel derived thin film biosensor.
A dual-fractal analysis is required to adequately describe the binding kinetics. A single-fractal
analysis is adequate to describe the dissociation kinetics. The values of (a) the binding rate
coefficient,
k
, and the fractal dimension,
D
f
, for a single-fractal analysis, (b) the dissociation