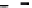Biomedical Engineering Reference
In-Depth Information
0.8
0.6
0.4
0.2
0
0
100
200
300
400
500
600
Time (s)
Figure 8.4
Binding of 50 ng/mL myoglobin in serum to antimyoglobin antibody immobilized on a SPR
biosensor surface (
Masson et al., 2007
). When only a solid line (--) is used then a single-fractal
analysis applies. When both a dashed (- - -) and a solid (--) line are used then the dashed line
represents a single-fractal analysis and the solid line represents a dual-fractal analysis.
Tables 8.2
and
8.3
. It is of interest to note that as the fractal dimension increases by a factor
of 1.39 from a value of
D
f1
equal to 1.9884 to
D
f2
equal to 2.7716, the binding rate coeffi-
cient increases by a factor of 5.78 from a value of
k
1
equal to 0.0541 to
k
2
equal to
0.3129. It is seen that changes in the fractal dimension or the degree of heterogeneity on
the biosensor surface and in the binding rate coefficient are in the same direction.
Yang et al. (2007)
report that cardiac hypertrophy is an independent risk factor for the develop-
ment of heart failure and sudden cardiac death (
Levy et al., 1990; Ruskoaho, 1992
). In cardiac
hypertrophy there is an increase in heart size and/or myofibrillar volume without a change
in myocyte number (
Yang et al., 2007
).
Lovell and Carabello (2000) and Diane et al. (2000)
point out that the cell morphology (cell size increases and there is myofibrillar re-organization)
is altered.
Yang et al. (2007)
report that there is a need to monitor cardiac myocyte hypertrophy
noninvasively and in real time. These authors have developed a novel microfluidic impedance
assay for monitoring endothelin-induced cardiomyocyte hypertrophy.
Yang et al. (2007)
developed and fabricated a DEP microfluidic device. This device is capa-
ble of concentrating cells from a dilute sample to form a confluent cell monolayer on a
microelectrode surface. This device, the authors claim can increase the sensitivity of their
impedance system besides significantly reducing the detection time required. They also
treated their cardiomyocytes with ET-1, which is a known hypertrophic agent.
Figure 8.5a
shows the binding and dissociation of the cardiac myocytes (heart muscle
cells) in the presence of 4.6
10
3
mL
1
of ET-1 and on using the DEP device to the
microelectrodes (comprising the impedance assay). A single-fractal analysis is adequate to
describe the binding and the dissociation kinetics. The values of (a) the binding rate coeffi-
cient,
k
, and the fractal dimension,
D
f
, for a single-fractal analysis, and the dissociation rate
coefficient,
k
d
, and the fractal dimension for dissociation,
D
fd
, for a single-fractal analysis are
given in
Tables 8.2
and
8.3
. In this case the affinity,
K
(
¼