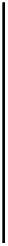Biomedical Engineering Reference
In-Depth Information
3E-07
1.4E-07
1.2E-07
2.5E-07
1E-07
2E-07
8E-08
1.5E-07
6E-08
4E-08
1E-07
5E-08
2E-08
0
0
0
2000
4000
6000
8000
0
1000
2000
3000
4000
5000
6000
A
B
Time (s)
Time (s)
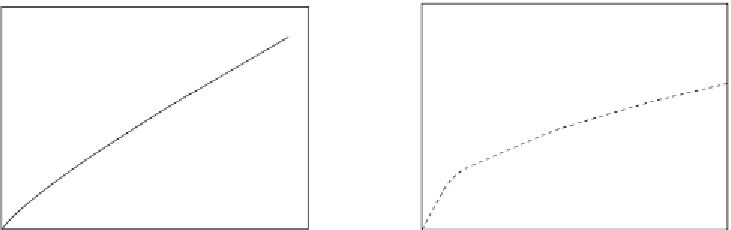
Figure 7.12
Binding of 500 mM glucose in solution to screen-printed water-based (WB) CoPC (cobalt
phthlocyanine) (electrocatalyst) microband biosensor (Pemberton et al., 2008). Influence of
quiescent and stirred conditions: (a) Quiescent (b) stirred.
Figure 7.12a
shows the binding of 500 mM glucose to a WB (water based)-CoPC (cobalt
phthalocyanine)-GOD (glucose oxidase) microband electrode at pH 8.0 (phosphate buffer)
under quiescent conditions (
Pemberton et al., 2009
). A single-fractal analysis is adequate
to describe the binding kinetics. The values of the binding rate coefficient,
k
, and the fractal
dimension,
D
f
, for a single-fractal analysis are given in
Table 7.8
.
Figure 7.12b
shows the binding of 500 mM glucose in solution to a WB-CoPC-GOD
microband electrode at pH 8.0 under stirred conditions (
Pemberton et al., 2009
). A dual-frac-
tal analysis is required to adequately describe the binding kinetics. The values of (a) the bind-
ing rate coefficient,
k
, and the fractal dimension,
D
f
, for a single-fractal analysis, and (b) the
binding rate coefficients,
k
1
and
k
2
, and the fractal dimensions,
D
f1
and
D
f2
, for a dual-fractal
analysis are given in
Table 7.8
.
Table 7.8: Binding of 500 mM glucose in solution to WB (water-based)-CoPC (cobalt
phthalocyanate)-GOD (glucoseoxidase) microband biosensor at pH 8.0 (phosphate buffer)
(
Pemberton et al., 2009
).
Experimental
Condition
k
k
1
k
2
D
f
D
f1
D
f2
7.110
11
Quiescent
na
na
1.3316 0.07396
na
na
0.710
11
10
9
10
11
10
9
Stirred
3.6
7.0
1.2
0.0 2.0848
0.2608
1.0232
1.7414
10
9
10
11
2.2
0.9
0.1814
0.0606
Influence of stirring.