Biomedical Engineering Reference
In-Depth Information
350
300
250
200
150
100
50
0
0
100
200
300
400
500
600
Time (s)
Figure 6.2
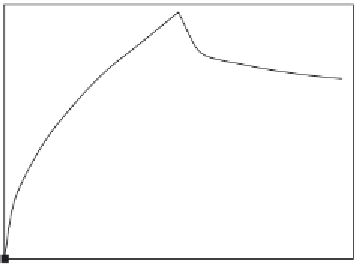
Binding and dissociation of the E. coli to the aptamer antibody-conjugated magnetic bead
biosensor, and amplified by real-time PCR (
Lee et al., 2009
).
concentration is low. Note also that the fractal dimension exhibited during the binding of 10
8
CFU/ml
E. coli
in solution to the antibody-conjugated magnetic beads is higher by 18% when
compared with the fractal dimension exhibited during the binding of 10
7
CFU/ml in solution
to the immunosensor. Note that the fractal dimension is based on a log scale and even small
changes in the value of the fractal dimension represent significant changes in the degree of
heterogeneity on the biosensor surface.
The heterogeneity on the biosensor surface could be the result of various factors. They could
include (among others) the heterogeneity of the sensor surface itself, heterogeneities due to
the receptors on the surface, or the heterogeneities that arise during the binding of the analyte
or in the analyte itself. No attempt is made here to delineate these different causes, or identify
them, except to point out that all of these possible heterogeneities on the sensing surface are
combined together and described by a single value, the fractal dimension,
D
f
.
Centi et al. (2008)
have recently developed an electrochemical aptamer-based assay coupled to
magnetic beads for the detection of thrombin. These authors developed a direct and an indirect
competitive assay by immobilizing both an aptamer and a protein. Electrochemical transduc-
tion coupled with innovative use of magnetic beads was used. These authors were able to
achieve a detection limit of 430 nM of thrombin. Also, these authors were able to achieve a
lower limit of 175 nM by detecting the product catalyzed enzymatically by thrombin.
Centi et al. (2008)
indicate that aptamers are nucleic acids that may be generated against
amino acids, drugs, proteins, and molecules. SELEX (systematic evolution of ligands by
experimental enrichment), an iterative procedure that uses binding, separation, and amplifica-
tion may be used to isolate these aptamers.
Centi et al. (2008)
point out that though aptamers
have appeared in recent literature (
Ellington an Szostak, 1990; Tuerk and Gold, 1990;