Biomedical Engineering Reference
In-Depth Information
N
O
O
N
N
AA
4
AA
5
AA
6
NH
2
O
H
NH
O
O
34
H
2
N
35
NH
HN
AA
1
AA
2
AA
3
NH
HN
NHAc
dye
AA
1-3
=
Gly, Ala, Val, Phe, Gln
AA
4-6
=
O
Gly, Ala, Ser, Met, His
NH
O
5 × 5 × 5 × 5 × 5 × 5 = 15,625 receptors
Fig. 10 Nonsymmetric tweezer receptor library 34 with a guanidinium template for carboxylate
recognition and dye labeled substrate N-Ac-Lys(dye)-
D
-Ala-
D
-Ala-OH 35
oxoanion binding motif. By means of UV/Vis experiments, a binding constant of
3
10
3
M
1
was determined for the complexation between 33 and the polar
tripeptide N-Ac-Lys-
D
-Ala-
D
-Ala-OH in buffered water at neutral pH. Weaker
binding, by a factor of 5, (
K
10
2
M
1
) was observed for 32. While the
main driving force for complex formation stems from the interaction between the
free C-terminus of the tripeptide with the GCP moiety of the host systems,
the difference between the two tweezer receptors could be attributed to additional
non-covalent interactions between the substrate and 33, such as an additional salt
bridge between the aspartic acid, which is negatively charged at this pH, and the
ammonium group of the substrate's lysine. Furthermore, in contrast to 32, the
hydroxyl groups in the serine and tyrosine side chains of the first arm of 33 are
potential hydrogen bond donor and acceptor sites and might thus further stabilize
the complex via additional hydrogen bonds. In conclusion, these two artificial
receptors are illustrative examples of the crucial role of the building blocks in the
interaction between host and guest, which can only form a stable complex if the
building blocks are appropriate for the formation of non-covalent interactions
between the two molecules.
Kilburn prepared the combinatorial tweezer receptor library 34, depicted in
Fig.
10
, with 15,625 (5
6
) members [
23
]. The two nonsymmetrical side chains
consisted of varying tripeptide sequences. A guanidinium headgroup served as
both the scaffold and as a carboxylate binding site in order to increase the binding
affinity to the C-terminus of the substrate via a hydrogen bond-enforced salt bridge.
The positive influence of the cationic headgroup was confirmed by comparing
receptors that were bound to the solid support directly via the guanidine [
24
].
These model systems lost all of their affinity to the substrate because the guanidine
could not be protonated under these circumstances. An on-bead screening of 34
with the dye labeled peptide N-Ac-Lys(dye)-
D
-Ala-
D
-Ala-OH (35) in buffered
water at pH 8.75 revealed that less than 2% of the library members bound the
substrate. The selected hit sequences were highly consistent, as shown in Table
1
.
Additional on-bead binding studies with the best receptor of the sequence AA
1-6
¼
6
¼
Gly-Val-Val-Met-His-Ser showed a binding constant of 10
3
M
1
. The corresponding
diastereomeric substrate N-Ac-Lys(dye)-Ala-Ala-OH was bound less efficiently with
a binding constant of 3
10
2
M
1
. Binding studies in solution did not lead to











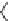


























Search WWH ::

Custom Search