Biomedical Engineering Reference
In-Depth Information
HO
O
NHAc
O
NHAc
NH
NH
H
N
O
O
H
N
O
O
NH
NH
O
O
HO
OH
NH
NH
H
2
N
H
2
N
N
H
H
2
N
H
2
N
N
H
O
O
NH
.
HCl
O
NH
.
HCl
O
O
HN
HN
O
HN
O
HN
H
N
N
32
N
H
33
NH
O
O
O
O
HCl
.
H
2
N
Fig. 9 Tweezer receptors 32 and 33 featuring two nonidentical peptidic arms—one of them is
equipped with a guanidiniocarbonyl pyrrole oxoanion binding motif
approach. One of the two libraries contained the template without change, for the
other the template was derivatized with a hydrocarbon chain, as illustrated in Fig.
8
,
to reduce its flexibility. Both libraries were then tested for their binding affinity
towards the dansyl-labeled biologically relevant substrates Gly-
D
-Ala-
D-
Ala-OH
(29) and Gly-
D
-Ala-
D
-Lac-OH (30) in aqueous phosphate buffer. Both substrates
were linked to the fluorescent dansyl group via a glycine spacer. Characterization of
hit structures revealed a highly consistent distribution of amino acids at position 3.
For the dipeptide 29, lysine was reported in 40% of cases and proline and alanine
each accounted for a further 20%, which shows that these amino acids are thus
clearly preferred with respect to a merely statistical distribution (ca. 3%). The
results from the screening for the corresponding depsipeptide, 30, showed that
position 3 was mainly occupied by proline (50%), followed by lysine (25%) and
alanine (20%). The conformity was less well pronounced for positions 1 and 2.
However, in both cases mainly aromatic or aliphatic amino acids were present,
which might be due to interaction of the nonpolar amino acid side chains with the
dansyl label. The glycine linker is probably too short to prevent this unwanted
hydrophobic interaction with the fluorescence dye. With a binding constant of
5
10
2
M
1
the highest affinity for the depsipeptide in aqueous phosphate buffer
was found for the amino acid sequence AA
1-3
L
-Leu-
L
-Phe-
L
-Lys. In chloroform
binding constants were significantly higher with values of up to 10
4
M
1
. Due to the
competitive influence of water, the achieved binding constants are rather low. The
comparison between both libraries reveals that the hydrocarbon chain has no
significant influence on the complex stability. The addition of a third arm, which
was indirectly attached to the scaffold via the hydrocarbon chain (31), did neither
improve binding affinity nor selectivity.
Schmuck developed the two tweezer receptors 32 and 33, depicted in Fig.
9
[
21
,
22
]. Based on the work of Liskamp, the aromatic scaffold 22 (Fig.
7
) was used
to bridge two peptidic arms. In order to synthesize receptors which feature two
nonidentical arms, such as 32 and 33, the scaffold was equipped with two orthogo-
nal protecting groups (Fmoc, Boc). One of the receptor's arms consisted of three
amino acids while the other featured two amino acids plus a GCP group as an
¼
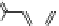




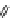






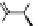





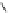

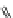







































































Search WWH ::

Custom Search