Biomedical Engineering Reference
In-Depth Information
HN
AA
1-4
= Ala, Val, Leu, Phe, Pro,
Ser, Thr, Lys, Asp, Glu
O
C7
C3
O
10 × 10 × 10 × 10 = 10,000 receptors
Gly
AA
3
AA
4
NHAc
O
13
Gly
AA
1
AA
2
NHAc
Fig. 5 The peptidosteroidal tweezer receptor library 13 with two differently substituted side
chains was simultaneously screened for two substrates labeled with either a
blue
or a
red dye
O
R
2
H
N
H
N
H
N
AA
1
AA
2
AA
3
N
H
NHAc
O
O
Disperse Red 1
R
3
N
H
O
O
14
H
N
15
AA
1-3
=
O
O
Gly, L/D: Ala, Val,
Leu, Phe, Pro, Ser,
Thr, Asp, Glu, Asn,
Gln, His, Lys, Arg
H
N
O
O
NH
O
O
R
3
Disperse Red 1
H
N
N
H
N
H
29 × 29 × 29 = 24, 389 substrates
O
R
2
NH
2
N
H
2
NH
2
H
2
N
O
H
2
N
H
N
O
O
NN
5
H
N
H
2
N
O
NH
2
H
2
N
NH
2
NH
2
16
17
18
19
20
Fig. 6 Schematic design of tweezer receptors 15 containing two identical tripeptidic arms labeled
with a
red dye
(
left
) and alternative templates 16-20 (
right
)
the rigid steroidal skeleton, binding strength was weakened by a factor of five and
complete loss of selectivity was observed. In terms of the ability to recognize the
pentapeptidic substrates, highly flexible receptors seem to be disadvantageous,
probably due to a loss of pre-organization.
Wennemers synthesized five closely related, yet distinct, tweezer receptors 15,
which were labeled with a red dye, as depicted in Fig.
6
. The two identical arms
were linked via a rigid diamino diketopiperazine template [
17
]. Screening for
substrate selectivity in chloroform was carried out with the help of a substrate
library comprising 29
3
24,389 tripeptides (14). Binding constants of up to
10
3
M
1
(chloroform) and high selectivity were observed: only 1 out of 5,000
sequences was bound, e.g., the receptor with the amino acid sequence
L
-Tyr-
L
-Asn
(Trt)-
L-
Phe exclusively selects peptides containing
D
-His followed by two hydro-
phobic
D
-amino acids. In order to verify whether a simplified receptor design is still
¼




















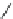
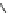












































































Search WWH ::

Custom Search