Biomedical Engineering Reference
In-Depth Information
O
H
O
N
O
O
AA
1
AA
2
AA
3
HN
H
Lys
Gly
Gly
Gly
Gly
Lys
NHAc
O
NH
O
NH
25
OH
O
O
O
NH
NH
2
HN
AA
1-3
=
Ala, Asp, Glu, Phe, Gly, His, Ile,
Lys, Leu, Met, Asn, Pro, Gln,
Arg, Ser, Thr, Val, Trp, Tyr
HN
NH
2
HN
O
O
N
O
NH
2
NH
O
NH
NH
HN
19 × 19 × 19 = 6,859 receptors
N
NH
O
O
NH
O
O
HN
O
O
NH
AA
1
=
A
r
g
AA
2
=
Lys
AA
3
= Val
AA
1
=
Arg
AA
2
=
Lys
AA
3
=
Gly-Gly-Glu-Lys-Tyr-Leu
N
NH
H
28
NH
26
27
29
Fig. 11 Combinatorial library of 6,859 (19
3
) ATP receptors 25 and hit structure 26. Rationally
improved compound 27 and crosslinked ATP selective receptor 29 with phosphate (
blue
) and
nucleobase (
red
) binding site. Cyclization was achieved by crosslinking the first and last Lys
residue with dimethyl adipimidate (
green
)
Matsui derived his ATP selective receptor 29 (Fig.
11
) in a three-step procedure
[
17
]. Firstly, the nonapeptide library 25, with three combinatorial amino acid
positions and comprising 6,859 (19
3
) members was screened with the help of
fluorescently labeled ATP. The binding constant of the best receptor 26, with
AA
1-3
¼
Arg-Lys-Val, was determined on-bead in buffered water at pH 7 to be
10
3
M
1
. Molecular modeling revealed that Arg and Lys bound to the phos-
phate hinge, while there is no interaction with the nucleobase. Furthermore, Val
does not seem to take part in the recognition process. Thus Val was replaced by
Gly-Gly-Glu-Lys-Tyr-Leu, a sequence derived from the adenine binding site of
biotin carboxylase. The corresponding receptor 27 had an increased affinity to ATP
(
K
6
10
4
M
1
). In the last step, the thus-derived peptide was crosslinked with
dimethyl adipimidate (28) in the presence of ATP.
The cyclic peptide 29 now had a binding constant of 5
¼
10
4
M
1
to ATP.
Additionally, in contrary to its precursors, 29 was now able to distinguish between
ATP and ADP, AMP, GDP, GTP which are all bound significantly weaker by one
order of magnitude. When the cyclization was carried out without the presence of
ATP the affinity of the corresponding receptors was decreased, which suggests the
formation of alternative cyclization products. This effect was attributed to the two
inner Lys moieties being involved with binding to the phosphate hinge. Only the
first and the last lysine side chains are then freely available for crosslinking.
Unfortunately, the authors did not provide additional data obtained in solution to
support this hypothesis. However, they were able to prove the general concept of
combining combinatorial chemistry with rational design and molecular imprinting
in order to increase the affinity of a library-derived receptor—in this case by one
order of magnitude.
Schmuck developed the tweezer receptor 30 (Fig.
12
) with two symmetric
peptidic arms which are connected via an aromatic template and contain phenylal-
anine, lysine and a guanidiniocarbonyl pyrrole (GCP) oxoanion binding site as the
























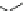




















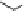






































Search WWH ::

Custom Search