Biomedical Engineering Reference
In-Depth Information
OH
H
O
O
O
OH
(OH)
14
O
(OH)
14
OH
H
O
O
OH
HO
HO
O
H
N
(OH)
6
(OH)
14
OH
O
O
H
N
(OH)
6
OH
NH
(OH)
14
O
OH
O
OH
(OH)
14
(OH)
6
NH
OH
OH
HN
OH
O
OH
H
N
O
OH
(OH)
6
O
O
OH
(OH)
6
HO
O
22
OH
Fig. 9 Receptor 22 combines four cyclodextrin units with two guanidinium scaffolds
interior of the cavity is not hydrophobic, it is less hydrophilic than water. Thus 21
forms strong nucleotide-complexes, with binding constants of up to 3
10
6
M
1
with ATP (effective binding constant obtained by potentiometry, verified via NMR
titration). This is stronger by more than one order of magnitude compared to its
complex with AMP. However, this host system is not able to differentiate between
nucleobases or between ribose and deoxyribose sugar moieties. The receptor 20,on
the other hand, forms weaker complexes (e.g., 10
5
M
1
for ATP) but is able to
differentiate both between nucleobases and sugars.
With the help of knockout analogs (e.g., ribose, phosphoribose, phosphate) the
different contributions to the overall binding constant could be assigned. The main
driving force stems from ionic interactions between phosphate and methylammonium
groups. Surprisingly, the nucleobase does not contribute in an advantageous way to
the binding. Instead, the second most important contribution to the binding strength
stems from interactions with the sugar moiety. With the help of NMR and molecular
modeling it could be shown that the ribose resides inside the CyD cavity and interacts
with it via hydrogen bonds. This is also why the deoxyribose is bound more weakly
than ribose, despite the fact that it is more hydrophobic. The nucleobase-selectivity of
20 was explained by secondary, rather weak hydrogen bonds between nucleobase and
upper rim hydroxyl groups. The higher charged 21 pulls the nucleotide deeper into
the cavity and thus prevents these secondary interactions from forming. Again, these
experiments are excellent examples of the difficult task of designing receptors which
have high selectivity as well as high affinity to a given substrate. All too often these
two desirable properties are antipodal. Furthermore Schneider's work is an instruc-
tive example on how model systems help to improve our understanding of anion
recognition.
Marsura made use of the same principle by bridging four
-cyclodextrin cavities
via two guanidinium groups to receptor 22, as depicted in Fig.
9
[
14
]. The cationic
scaffold allows for hydrogen bonded ion pairing to nucleotide phosphates while the
CyDs display pockets for hydrophobic inclusion. NMR experiments in D
2
OatpH6.5
revealed that 1:2 host-guest complexes are formed to AMP, ADP, and ATP with
similar binding constants of 2
b
10
6
M
2
. Two substrate molecules are bound with
similar binding constants, representing a noncooperative binding mode with two
independent complexation steps. NMR also revealed that the phosphate hinge

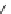



































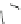









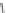





































Search WWH ::

Custom Search