Biomedical Engineering Reference
In-Depth Information
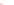
NH
2
N
H
2
19
NH
2
NH
2
O
O
NH
2
H
2
N
O
O
R
R R
R
O
O
NH
2
O
O
H
2
N
R
4
O
O
H
2
N
R =
O
O
O
O
NH
2
Fig. 7 Calixarene-based receptor 19 with four phenylamidinium groups
OH
NH
O
OH
O
O
H
O
O
NH
NH
O
O
OH
HO
HO
OH
O
O
O
H
NH
HO
HO
HO
H
O
O
O
OH
OH
O
O
O
O
N
OH
OH
HO
O
O
OH
OH
OH
OH
O
O
OH
OH
H
H
OH
OH
O
O
OH
O
O
OH
OH
OH
O
O
O
O
OH
OH
HO
HN
O
O
HN
OH
(OH)
14
(OH)
14
20
21
(OH)
5
(NHMe)
2
(NHMe)
7
Fig. 8 Cyclodextrin-based receptors 20 and 21 with two and, respectively, three methylammonium
groups
for the accommodation of nonpolar guest molecules. NMR titrations in buffered
D
2
O at pH 8.3 revealed affinities corresponding to the charge on the nucleotide
guest
(ATP
>
ADP
>
AMP
>
cAMP) and binding constants
for
the 1:1
10
5
M
1
. AMP was slightly preferred over
the G, C, T, and U derivatives by a factor of 2-3. As derived from NMR and
molecular modeling the nucleobase is bound within the hydrophobic bowl and the
phosphate interacts with two phenylamidinium groups via hydrogen bonded salt
bridges. The preference for A-nucleotides can be ascribed to a superior
stereoelectronic complementarity of this nucleobase with regard to its inclusion
into the bowl-type cavity.
Another sort of cavity-forming molecules was used as a scaffold by Schneider
[
13
]. By exchanging two or, respectively, all seven 6-hydroxy groups in
host-guest complexes of up to 7
-cyclo-
dextrin (CyD) for aminomethyl moieties, the two nucleotide receptors 20 and 21,
shown in Fig.
8
, were obtained. CyDs are highly water soluble, cyclic, 1,4-linked
oligo-
b
-
D
-glucopyranoside units. Their topology is toroidal, with a larger and a
smaller opening which present their hydroxyl groups to the solvent. Although the
a
























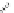

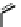














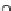
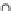
























































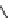









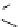




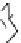
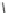























































































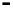
Search WWH ::

Custom Search