Biomedical Engineering Reference
In-Depth Information
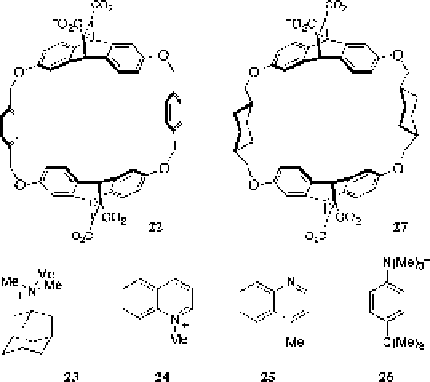
Fig. 12 Macrocyclic hosts that mimic aromatic cage motifs, and their guests that have been used
to understand the nature of their interactions
It has always been a confounding element of these motifs (natural and synthetic)
that a hydrophobic component (and not the cation-pi interaction) might be suffi-
cient to drive the observed binding of quaternary ammonium ions (which are much
more hydrophobic than primary ammonium ions). But comparisons in water of
nearly isostructural guests such as 24 and 25 that bear different charges revealed a
significant difference in affinities that can only be explained by the multiple
cation-pi interactions that exist between the guest 24 and the cyclophane. Further,
the guest 26, with isosteric aliphatic -C(Me)
3
and ammonium -N(Me)
3
+
ends,
shows a strong preference for binding with the charged (and slightly less hydro-
phobic) -N(Me)
3
+
end inside the aromatic cage of 22; if hydrophobicity were the
prime driver for guest binding, then the more hydrophobic -C(Me)
3
end would win
out. Finally, the host analog 27, with two of the benzene rings of 22 replaced with
cyclohexane walls that are more hydrophobic and more polarizable than the
benzenes in the parent host, also shows decreased affinities for ammonium ion
guests that support the key role of cation-pi interactions in the parent host 22.
Other informative comparisons have been conducted using natural, protein-
based aromatic cage motifs as “receptors” themselves for physical organic studies.
In one kind of study, strong-binding quaternary ammonium ion ligands of the type
-N(Me)
3
+
(31) are compared to ligand analogs that are isostructural except for -C
(Me)
3
substitutions (32, Fig.
13
)[
60
,
61
]. The affinities in both studies are higher
for the charged species that can form multiple cation-pi interactions with their
protein binding partners. In a different kind of study, the protein itself is altered to
reduce the strength of the proposed cation-pi interaction by installation of unnatural
aromatic amino acids with decreased electron density relative to the native Trp
residues (e.g., F-Trp, F
2
-Trp, F
3
-Trp) [
62
]. Again, the dominant role of certain
cation-pi contacts in these biologically important recognition events is supported
Search WWH ::

Custom Search